よむ、つかう、まなぶ。
資料1-2 調査結果報告書 (27 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_24579.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和3年度第31回 3/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
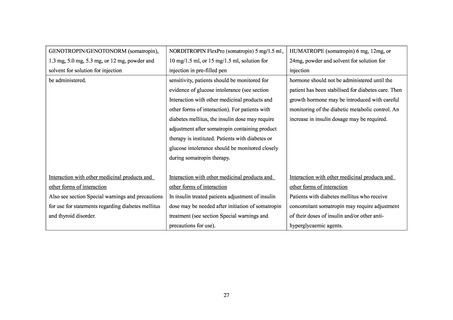
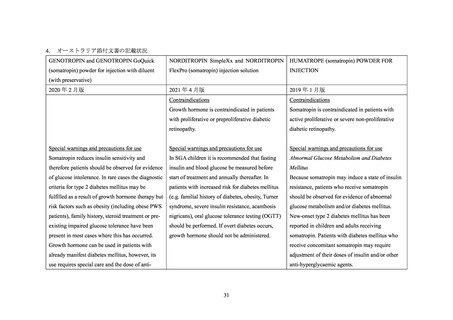
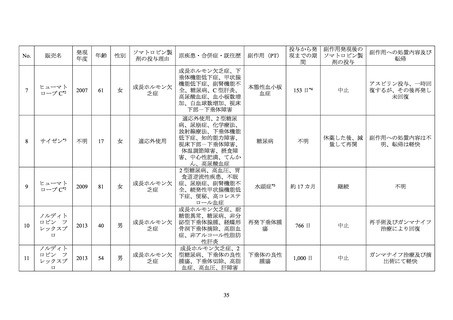
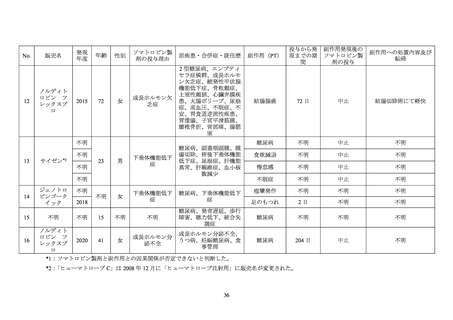
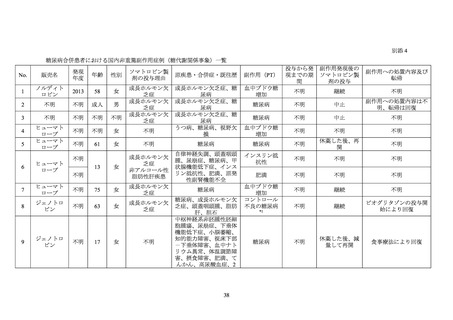
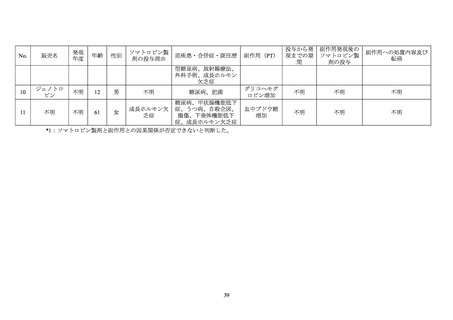
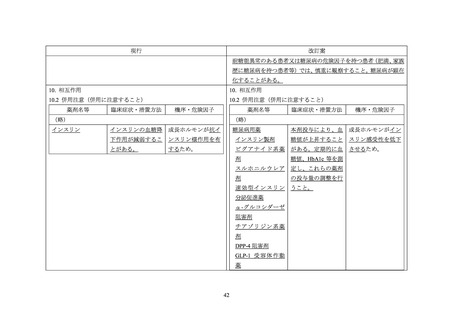
GENOTROPIN/GENOTONORM (somatropin),
NORDITROPIN FlexPro (somatropin) 5 mg/1.5 ml ,
HUMATROPE (somatropin) 6 mg, 12mg, or
1.3 mg, 5.0 mg, 5.3 mg, or 12 mg, powder and
10 mg/1.5 ml, or 15 mg/1.5 ml, solution for
24mg, powder and solvent for solution for
solvent for solution for injection
injection in pre-filled pen
injection
be administered.
sensitivity, patients should be monitored for
hormone should not be administered until the
evidence of glucose intolerance (see section
patient has been stabilised for diabetes care. Then
Interaction with other medicinal products and
growth hormone may be introduced with careful
other forms of interaction). For patients with
monitoring of the diabetic metabolic control. An
diabetes mellitus, the insulin dose may require
increase in insulin dosage may be required.
adjustment after somatropin containing product
therapy is instituted. Patients with diabetes or
glucose intolerance should be monitored closely
during somatropin therapy.
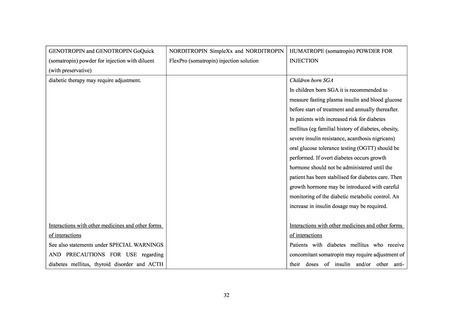
Interaction with other medicinal products and
Interaction with other medicinal products and
Interaction with other medicinal products and
other forms of interaction
other forms of interaction
other forms of interaction
Also see section Special warnings and precautions
In insulin treated patients adjustment of insulin
Patients with diabetes mellitus who receive
for use for statements regarding diabetes mellitus
dose may be needed after initiation of somatropin
concomitant somatropin may require adjustment
and thyroid disorder.
treatment (see section Special warnings and
of their doses of insulin and/or other anti-
precautions for use).
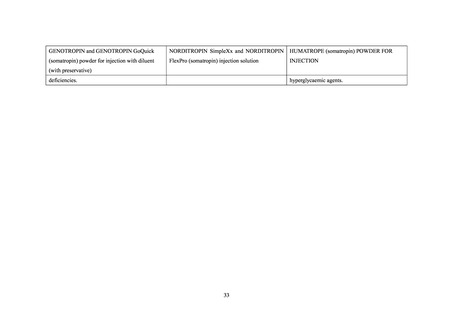
hyperglycaemic agents.
27
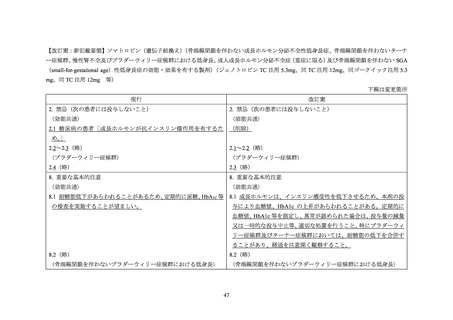
NORDITROPIN FlexPro (somatropin) 5 mg/1.5 ml ,
HUMATROPE (somatropin) 6 mg, 12mg, or
1.3 mg, 5.0 mg, 5.3 mg, or 12 mg, powder and
10 mg/1.5 ml, or 15 mg/1.5 ml, solution for
24mg, powder and solvent for solution for
solvent for solution for injection
injection in pre-filled pen
injection
be administered.
sensitivity, patients should be monitored for
hormone should not be administered until the
evidence of glucose intolerance (see section
patient has been stabilised for diabetes care. Then
Interaction with other medicinal products and
growth hormone may be introduced with careful
other forms of interaction). For patients with
monitoring of the diabetic metabolic control. An
diabetes mellitus, the insulin dose may require
increase in insulin dosage may be required.
adjustment after somatropin containing product
therapy is instituted. Patients with diabetes or
glucose intolerance should be monitored closely
during somatropin therapy.
Interaction with other medicinal products and
Interaction with other medicinal products and
Interaction with other medicinal products and
other forms of interaction
other forms of interaction
other forms of interaction
Also see section Special warnings and precautions
In insulin treated patients adjustment of insulin
Patients with diabetes mellitus who receive
for use for statements regarding diabetes mellitus
dose may be needed after initiation of somatropin
concomitant somatropin may require adjustment
and thyroid disorder.
treatment (see section Special warnings and
of their doses of insulin and/or other anti-
precautions for use).
hyperglycaemic agents.
27