よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (8 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
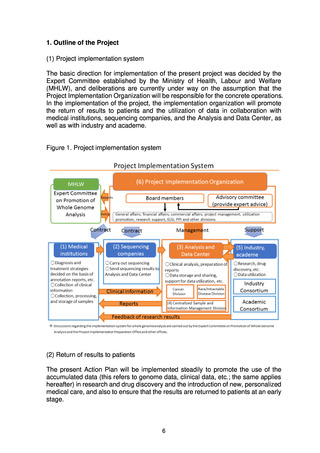
The information that can be returned to patients, and the specific ways in which it
can be returned, are envisaged as follows.
(a) Information relating to the results of research and drug discovery
New results obtained through research or drug discovery that uses the data
accumulated by the present project will be made available as appropriate to
industry and academe to promote the use of the Analysis and Data Center.
Generalized information on these results will be made available to the general
public over the Internet, etc.
(b) Information that can be introduced into clinical practice
Information relating to the condition that prompted a medical examination
• Information from the analysis results that is useful for medical care of the
patient, such as pathogenic variants determined at the time of whole genome
analysis to be of medical significance, will be returned to the patient promptly
to the greatest extent possible.
• With information that is not determined at the time of whole genome analysis
to be of medical significance, but is subsequently determined to be medically
significant as a result of advanced cross-sectional analysis, etc., efforts will be
made to return this information to the patient in line with the patient’s wishes
if it is judged to be useful for the treatment of the patient following discussions
by an expert panel.
Information not relating to the condition that prompted a medical examination
• Among the information on germline pathogenic variants, etc. that is
determined at the time of whole genome analysis to be of medical significance,
the information that can affect the health of the patient, despite being
unconnected to the patient’s current medical condition, is to be returned to the
patient in line with the patient’s wishes following discussions by an expert
panel, and with proper considerations such as provision of genetic counseling.
• Of the information on germline pathogenic variants, etc. that is not determined
at the time of whole genome analysis to be of medical significance but is
subsequently determined to be medically significant as a result of advanced
cross-sectional analysis, etc., the information that can affect the health of the
patient, despite being unconnected to the patient’s current medical condition,
is to be returned to the patient in line with the patient’s wishes following
discussions by an expert panel, and with proper considerations such as
provision of genetic counseling.
(c) Information relating to new personalized medical care
Systems will be put in place to enable as many patients as possible to have
opportunities to take part in clinical research or clinical trials that use the
results of whole genome analysis.
Figure 2. Information that can be returned to patients
Information that Can Be Returned to Patients and How It Is Returned
1. Information relating to the results of research and drug discovery
• Provision of novel therapeutic methods through medical product development*1
7
can be returned, are envisaged as follows.
(a) Information relating to the results of research and drug discovery
New results obtained through research or drug discovery that uses the data
accumulated by the present project will be made available as appropriate to
industry and academe to promote the use of the Analysis and Data Center.
Generalized information on these results will be made available to the general
public over the Internet, etc.
(b) Information that can be introduced into clinical practice
Information relating to the condition that prompted a medical examination
• Information from the analysis results that is useful for medical care of the
patient, such as pathogenic variants determined at the time of whole genome
analysis to be of medical significance, will be returned to the patient promptly
to the greatest extent possible.
• With information that is not determined at the time of whole genome analysis
to be of medical significance, but is subsequently determined to be medically
significant as a result of advanced cross-sectional analysis, etc., efforts will be
made to return this information to the patient in line with the patient’s wishes
if it is judged to be useful for the treatment of the patient following discussions
by an expert panel.
Information not relating to the condition that prompted a medical examination
• Among the information on germline pathogenic variants, etc. that is
determined at the time of whole genome analysis to be of medical significance,
the information that can affect the health of the patient, despite being
unconnected to the patient’s current medical condition, is to be returned to the
patient in line with the patient’s wishes following discussions by an expert
panel, and with proper considerations such as provision of genetic counseling.
• Of the information on germline pathogenic variants, etc. that is not determined
at the time of whole genome analysis to be of medical significance but is
subsequently determined to be medically significant as a result of advanced
cross-sectional analysis, etc., the information that can affect the health of the
patient, despite being unconnected to the patient’s current medical condition,
is to be returned to the patient in line with the patient’s wishes following
discussions by an expert panel, and with proper considerations such as
provision of genetic counseling.
(c) Information relating to new personalized medical care
Systems will be put in place to enable as many patients as possible to have
opportunities to take part in clinical research or clinical trials that use the
results of whole genome analysis.
Figure 2. Information that can be returned to patients
Information that Can Be Returned to Patients and How It Is Returned
1. Information relating to the results of research and drug discovery
• Provision of novel therapeutic methods through medical product development*1
7