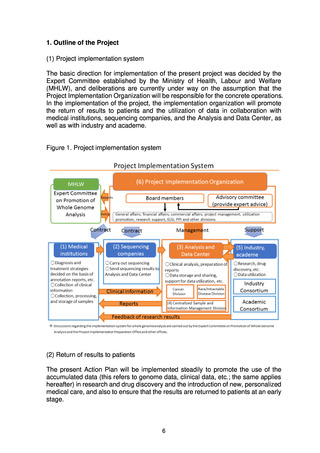
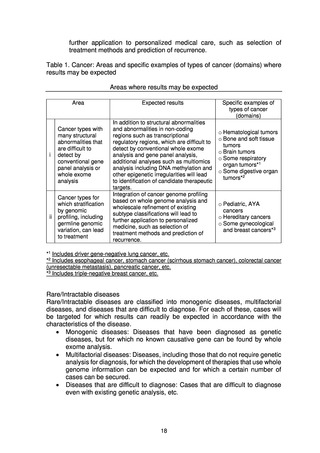
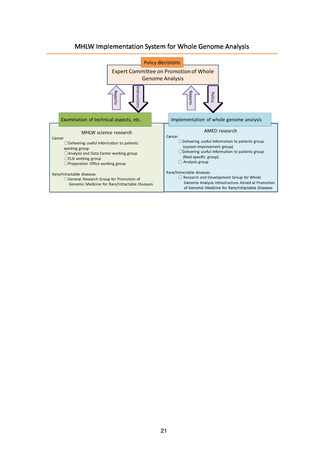
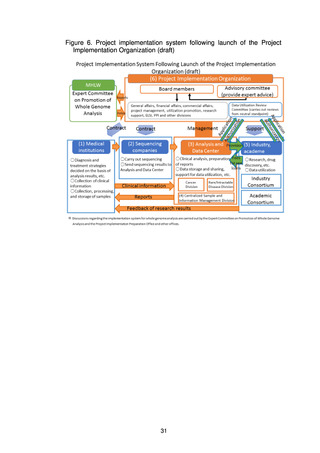
よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (3 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
0. Foreword
The Action Plan for Whole Genome Analysis (Version 1) (hereafter, Action Plan
[Version 1]) for cancer and rare/intractable diseases was formulated in December
2019 to promote whole genome analysis in Japan.
While the present project was affected by the global spread of COVID-19, in
FY2020, the government’s Basic Policy on Economic and Fiscal Management and
Reform 2020 (Cabinet decision of 17 July 2020) stipulated that the Action Plan for
Whole Genome Analysis was to be steadily promoted, and that systems were to be
put in place to enable extensive analysis and use of data by personnel from industry,
the government, and academe to provide new, personalized medical care to
patients for whom no treatment is available. On this basis, the relevant study groups
compiled policies for further promotion of the Action Plan (Version 1).
In FY2021, the previous study groups were organized into the Expert Committee
on Promotion of Whole Genome Analysis (tentative name) (hereafter, Expert
Committee), which was established within the Health Sciences Council Science and
Technology Committee as the top decision-making body for the Action Plan
(Version 1). In addition, to make steady progress with promotion of initiatives based
on the Action Plan (Version 1), at its meeting of June 2021, the Expert Committee
formulated the Action Plan for Whole Genome Analysis Roadmap 2021 (hereafter,
Roadmap 2021), which summarizes the items for implementation during FY2021
and FY2022.
Research using whole genome data has advanced globally over the last few years,
and from the perspective of facilitating research and drug discovery, as well as
protecting the genome information of citizens, the present project has taken on
greater significance in Japan. The Basic Policy on Economic and Fiscal
Management and Reform 2021 (Cabinet decision of 18 June 2021) stated that,
while taking into consideration the initiatives based on the Japan-U.S. Joint Leaders’
Statement, the Government would steadily promote the Action Plan for Whole
Genome Analysis and the Roadmap 2021 under the principle of putting patients
first and return of results to patients to provide new, personalized medical care to
patients for whom no treatment was previously available, and would promote the
creation of systems to enable extensive analysis and use of data by personnel from
industry, the government, and academe. The Expert Committee held discussions
on this basis, and from the perspective of steady promotion of whole genome
analysis, it decided to formulate the Action Plan for Whole Genome Analysis 2022
(hereafter, the present Action Plan).
The Basic Policy on Economic and Fiscal Management and Reform 2022 (Cabinet
decision of 7 June 2022) states that information infrastructure linking clinical
information to information such as the results of whole genome analysis is to be
constructed, and an environment for its utilization is to be put in place promptly to
promote drug discovery relating to cancer and rare/intractable diseases. On this
basis, the Action Plan specifies the directionality, in terms of the patients targeted
by the project and its implementation systems, over a period of around five years
starting from FY2022. In addition, it includes the management strategy with regard
2
The Action Plan for Whole Genome Analysis (Version 1) (hereafter, Action Plan
[Version 1]) for cancer and rare/intractable diseases was formulated in December
2019 to promote whole genome analysis in Japan.
While the present project was affected by the global spread of COVID-19, in
FY2020, the government’s Basic Policy on Economic and Fiscal Management and
Reform 2020 (Cabinet decision of 17 July 2020) stipulated that the Action Plan for
Whole Genome Analysis was to be steadily promoted, and that systems were to be
put in place to enable extensive analysis and use of data by personnel from industry,
the government, and academe to provide new, personalized medical care to
patients for whom no treatment is available. On this basis, the relevant study groups
compiled policies for further promotion of the Action Plan (Version 1).
In FY2021, the previous study groups were organized into the Expert Committee
on Promotion of Whole Genome Analysis (tentative name) (hereafter, Expert
Committee), which was established within the Health Sciences Council Science and
Technology Committee as the top decision-making body for the Action Plan
(Version 1). In addition, to make steady progress with promotion of initiatives based
on the Action Plan (Version 1), at its meeting of June 2021, the Expert Committee
formulated the Action Plan for Whole Genome Analysis Roadmap 2021 (hereafter,
Roadmap 2021), which summarizes the items for implementation during FY2021
and FY2022.
Research using whole genome data has advanced globally over the last few years,
and from the perspective of facilitating research and drug discovery, as well as
protecting the genome information of citizens, the present project has taken on
greater significance in Japan. The Basic Policy on Economic and Fiscal
Management and Reform 2021 (Cabinet decision of 18 June 2021) stated that,
while taking into consideration the initiatives based on the Japan-U.S. Joint Leaders’
Statement, the Government would steadily promote the Action Plan for Whole
Genome Analysis and the Roadmap 2021 under the principle of putting patients
first and return of results to patients to provide new, personalized medical care to
patients for whom no treatment was previously available, and would promote the
creation of systems to enable extensive analysis and use of data by personnel from
industry, the government, and academe. The Expert Committee held discussions
on this basis, and from the perspective of steady promotion of whole genome
analysis, it decided to formulate the Action Plan for Whole Genome Analysis 2022
(hereafter, the present Action Plan).
The Basic Policy on Economic and Fiscal Management and Reform 2022 (Cabinet
decision of 7 June 2022) states that information infrastructure linking clinical
information to information such as the results of whole genome analysis is to be
constructed, and an environment for its utilization is to be put in place promptly to
promote drug discovery relating to cancer and rare/intractable diseases. On this
basis, the Action Plan specifies the directionality, in terms of the patients targeted
by the project and its implementation systems, over a period of around five years
starting from FY2022. In addition, it includes the management strategy with regard
2