よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (40 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
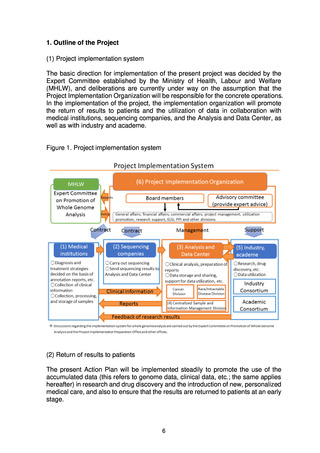
analysis, the status of clinical information collection, the status of return
of reports to medical institutions, etc.
The system infrastructure will be put in place and a prototype will be
completed in FY2022. The system will be upgraded in stages starting in
FY2023, with completion in about three years.
The following points should be noted in relation to the centralized
management system.
• From the perspective of preventing confusion over samples,
information on samples will always be managed in such a way that it
can be linked to the genome information and clinical information
databases.
• A common ID format for use when data are released will be examined.
• With regard to the consent from patients, the implementation of
technology to ensure traceability, such as confirmation of identity when
consent is obtained, deletion of data when consent is withdrawn, etc.
will be examined
• The system will be able to manage the starting point of each data set
(the point at which data from around 100 cases are registered
[excluding rare cancers]) in the Industry Consortium and the Academic
Consortium, and to enable centralized management of overview of data,
applications for use, granting of access rights, and status of use, etc.
by consortium members after the starting point is reached.
• The restricted period of the genome data and basic clinical information
that have been collected (a period greater than 24 months from the
starting point but not exceeding 30 months) will be managed, and data
that have passed the restricted period will be registered in a public
database.
• A mechanism will be established to enable samples from new patients
to be managed centrally using existing facilities (Centralized Sample
Management Center).
• A mechanism will be examined to enable information on samples to be
shared when necessary, such as when a patient participates in a
clinical trial.
• An automated system for storing and retrieving samples will be
examined.
• Security measures, etc. will be constantly upgraded.
• Linkage of the centralized management system to the system for
utilization of samples is essential, so that samples can be shared with
academe or industry if an application from a user is approved by the
utilization review committee and there is a request for utilization of
samples from the centralized management system. Even when
samples are outsourced to a sequencing company or stored at a
cooperating medical institution, cooperation in the utilization of samples
by the centralized management system is essential. The utilization of
samples will be conducted in accordance with the data utilization policy
and rules on data sharing (data sharing policy).
Various requirements for data management and system creation
39
of reports to medical institutions, etc.
The system infrastructure will be put in place and a prototype will be
completed in FY2022. The system will be upgraded in stages starting in
FY2023, with completion in about three years.
The following points should be noted in relation to the centralized
management system.
• From the perspective of preventing confusion over samples,
information on samples will always be managed in such a way that it
can be linked to the genome information and clinical information
databases.
• A common ID format for use when data are released will be examined.
• With regard to the consent from patients, the implementation of
technology to ensure traceability, such as confirmation of identity when
consent is obtained, deletion of data when consent is withdrawn, etc.
will be examined
• The system will be able to manage the starting point of each data set
(the point at which data from around 100 cases are registered
[excluding rare cancers]) in the Industry Consortium and the Academic
Consortium, and to enable centralized management of overview of data,
applications for use, granting of access rights, and status of use, etc.
by consortium members after the starting point is reached.
• The restricted period of the genome data and basic clinical information
that have been collected (a period greater than 24 months from the
starting point but not exceeding 30 months) will be managed, and data
that have passed the restricted period will be registered in a public
database.
• A mechanism will be established to enable samples from new patients
to be managed centrally using existing facilities (Centralized Sample
Management Center).
• A mechanism will be examined to enable information on samples to be
shared when necessary, such as when a patient participates in a
clinical trial.
• An automated system for storing and retrieving samples will be
examined.
• Security measures, etc. will be constantly upgraded.
• Linkage of the centralized management system to the system for
utilization of samples is essential, so that samples can be shared with
academe or industry if an application from a user is approved by the
utilization review committee and there is a request for utilization of
samples from the centralized management system. Even when
samples are outsourced to a sequencing company or stored at a
cooperating medical institution, cooperation in the utilization of samples
by the centralized management system is essential. The utilization of
samples will be conducted in accordance with the data utilization policy
and rules on data sharing (data sharing policy).
Various requirements for data management and system creation
39