よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (4 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
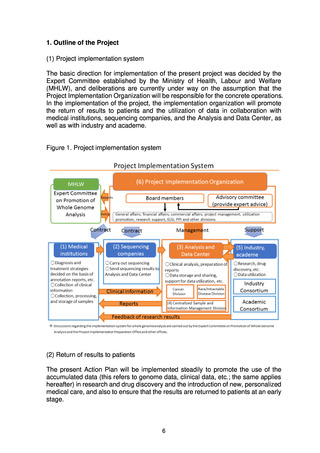
to return of results to patients and utilization, as well as items relating to ethical,
legal, and social issues (ELSI) and patient and public involvement (PPI).
The Expert Committee will continue its discussions in the future, taking into account
any changes in the surrounding environment and including the viewpoints of
patients’ families and citizens under the principle of putting patients first and return
of results to patients, with the aim of creating information infrastructure that links
clinical information to the results of whole genome analysis to promote drug
discovery relating to cancer and rare/intractable diseases. The Expert Committee
will create an environment for the use of this infrastructure at an early stage to
promote the utilization of the information in research and drug discovery and the
introduction of new, personalized medical care, while also steadily promoting the
prompt return of results to patients.
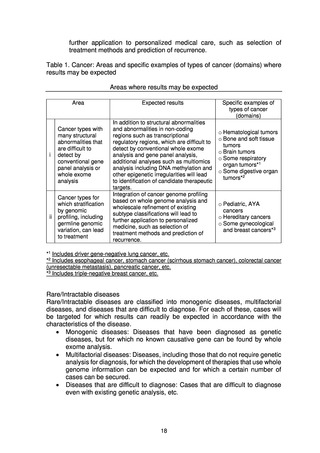
* In the present Action Plan, the term “cancer” refers to intractable cancers, rare
cancers, pediatric cancers, hereditary cancers, and other types of cancer for which
whole genome analysis is expected to have a certain effect, but for which the private
sector alone cannot conduct research and drug discovery.
3
legal, and social issues (ELSI) and patient and public involvement (PPI).
The Expert Committee will continue its discussions in the future, taking into account
any changes in the surrounding environment and including the viewpoints of
patients’ families and citizens under the principle of putting patients first and return
of results to patients, with the aim of creating information infrastructure that links
clinical information to the results of whole genome analysis to promote drug
discovery relating to cancer and rare/intractable diseases. The Expert Committee
will create an environment for the use of this infrastructure at an early stage to
promote the utilization of the information in research and drug discovery and the
introduction of new, personalized medical care, while also steadily promoting the
prompt return of results to patients.
* In the present Action Plan, the term “cancer” refers to intractable cancers, rare
cancers, pediatric cancers, hereditary cancers, and other types of cancer for which
whole genome analysis is expected to have a certain effect, but for which the private
sector alone cannot conduct research and drug discovery.
3