よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (35 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
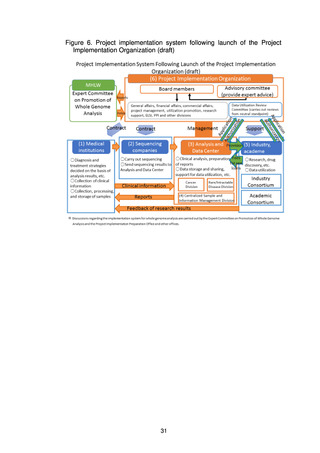
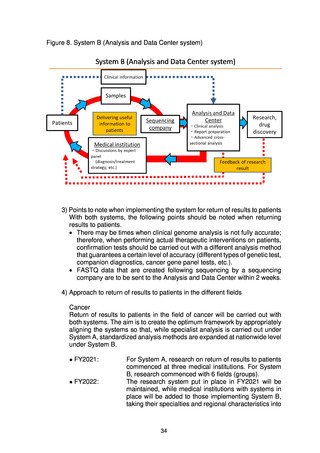
Figure 8. System B (Analysis and Data Center system)
3) Points to note when implementing the system for return of results to patients
With both systems, the following points should be noted when returning
results to patients.
• There may be times when clinical genome analysis is not fully accurate;
therefore, when performing actual therapeutic interventions on patients,
confirmation tests should be carried out with a different analysis method
that guarantees a certain level of accuracy (different types of genetic test,
companion diagnostics, cancer gene panel tests, etc.).
• FASTQ data that are created following sequencing by a sequencing
company are to be sent to the Analysis and Data Center within 2 weeks.
4) Approach to return of results to patients in the different fields
Cancer
Return of results to patients in the field of cancer will be carried out with
both systems. The aim is to create the optimum framework by appropriately
aligning the systems so that, while specialist analysis is carried out under
System A, standardized analysis methods are expanded at nationwide level
under System B.
● FY2021:
● FY2022:
For System A, research on return of results to patients
commenced at three medical institutions. For System
B, research commenced with 6 fields (groups).
The research system put in place in FY2021 will be
maintained, while medical institutions with systems in
place will be added to those implementing System B,
taking their specialties and regional characteristics into
34
3) Points to note when implementing the system for return of results to patients
With both systems, the following points should be noted when returning
results to patients.
• There may be times when clinical genome analysis is not fully accurate;
therefore, when performing actual therapeutic interventions on patients,
confirmation tests should be carried out with a different analysis method
that guarantees a certain level of accuracy (different types of genetic test,
companion diagnostics, cancer gene panel tests, etc.).
• FASTQ data that are created following sequencing by a sequencing
company are to be sent to the Analysis and Data Center within 2 weeks.
4) Approach to return of results to patients in the different fields
Cancer
Return of results to patients in the field of cancer will be carried out with
both systems. The aim is to create the optimum framework by appropriately
aligning the systems so that, while specialist analysis is carried out under
System A, standardized analysis methods are expanded at nationwide level
under System B.
● FY2021:
● FY2022:
For System A, research on return of results to patients
commenced at three medical institutions. For System
B, research commenced with 6 fields (groups).
The research system put in place in FY2021 will be
maintained, while medical institutions with systems in
place will be added to those implementing System B,
taking their specialties and regional characteristics into
34