よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (37 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
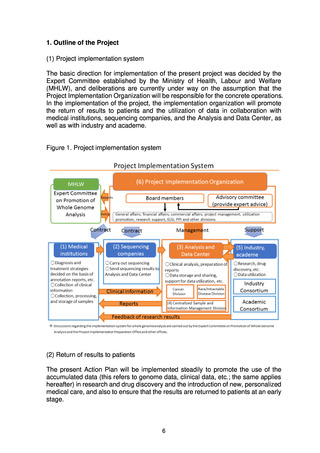
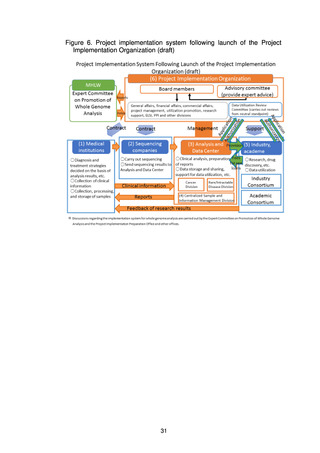
(b) Details of the system for return of results to patients
The basic approach to the various processes in the system for return of results
to patients (obtaining informed consent from patients; collection, processing,
transfer, and management of samples; sequencing; clinical analysis and
preparation of reports at the medical institution or the Analysis and Data
Center; and discussion of diagnosis, treatment strategy, etc. by the expert
panel at the medical institution) are set out below.
1) Obtaining informed consent from patients
Cancer
The Draft Model for the Action Plan for Whole Genome Analysis Explanatory
Document (hereafter, Draft Model),8 which has been approved by the Expert
Committee, will be used for the process of obtaining informed consent from
patients who are to participate in the present project. Participation in the
present project will be permitted only if a patient has consented to all items
included in the Draft Model.
The draft Model was drawn up for use in the AMED project Practical
Research for Innovative Cancer Control, and it provides a model of the items
that need to be included in the explanatory document as common items
relating to the Action Plan for Whole Genome Analysis. It should therefore be
noted that the Draft Model alone does not cover all the explanatory items
stipulated by the ethical guidelines, and individual medical institutions will
need to check carefully whether the explanatory document prepared using the
Draft Model contains the necessary items and also whether there are any
inconsistencies between the parts of the explanatory document that were
inserted from the Draft Model and the other parts.
The various different research groups will give their feedback on the Draft
Model, and a standardized Informed Consent Form (ICF) will be drawn up
during FY2022. The standardized ICF will be used from FY2023 onward.
Rare/Intractable diseases
Consent will be obtained using an Informed Consent Form based on the Draft
Model, with the addition of any extra explanation needed for individual
research studies.
o Introduction of information and communication technology (ICT) into the
informed consent process
The introduction of a system for obtaining informed consent (IC) by
electronic methods (e-consent) will be examined when the appropriate tools
and communication environment are in place. The advantages and points
to note regarding use of e-consent must be fully understood.
In addition, for the introduction of e-consent into medical settings, it will
be necessary to determine how hospitals transfer and manage IC
information and their means of cooperation with the Analysis and Data
Center by identifying specific problems in operability in medical settings and
8 Draft Model for the Action Plan for Whole Genome Analysis Explanatory Document
https://www.mhlw.go.jp/content/10901000/000904765.pdf
36
The basic approach to the various processes in the system for return of results
to patients (obtaining informed consent from patients; collection, processing,
transfer, and management of samples; sequencing; clinical analysis and
preparation of reports at the medical institution or the Analysis and Data
Center; and discussion of diagnosis, treatment strategy, etc. by the expert
panel at the medical institution) are set out below.
1) Obtaining informed consent from patients
Cancer
The Draft Model for the Action Plan for Whole Genome Analysis Explanatory
Document (hereafter, Draft Model),8 which has been approved by the Expert
Committee, will be used for the process of obtaining informed consent from
patients who are to participate in the present project. Participation in the
present project will be permitted only if a patient has consented to all items
included in the Draft Model.
The draft Model was drawn up for use in the AMED project Practical
Research for Innovative Cancer Control, and it provides a model of the items
that need to be included in the explanatory document as common items
relating to the Action Plan for Whole Genome Analysis. It should therefore be
noted that the Draft Model alone does not cover all the explanatory items
stipulated by the ethical guidelines, and individual medical institutions will
need to check carefully whether the explanatory document prepared using the
Draft Model contains the necessary items and also whether there are any
inconsistencies between the parts of the explanatory document that were
inserted from the Draft Model and the other parts.
The various different research groups will give their feedback on the Draft
Model, and a standardized Informed Consent Form (ICF) will be drawn up
during FY2022. The standardized ICF will be used from FY2023 onward.
Rare/Intractable diseases
Consent will be obtained using an Informed Consent Form based on the Draft
Model, with the addition of any extra explanation needed for individual
research studies.
o Introduction of information and communication technology (ICT) into the
informed consent process
The introduction of a system for obtaining informed consent (IC) by
electronic methods (e-consent) will be examined when the appropriate tools
and communication environment are in place. The advantages and points
to note regarding use of e-consent must be fully understood.
In addition, for the introduction of e-consent into medical settings, it will
be necessary to determine how hospitals transfer and manage IC
information and their means of cooperation with the Analysis and Data
Center by identifying specific problems in operability in medical settings and
8 Draft Model for the Action Plan for Whole Genome Analysis Explanatory Document
https://www.mhlw.go.jp/content/10901000/000904765.pdf
36