よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (2 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Table of Contents
0. Foreword ................................................................................................................................ 2
1. Outline of the Project ............................................................................................................ 6
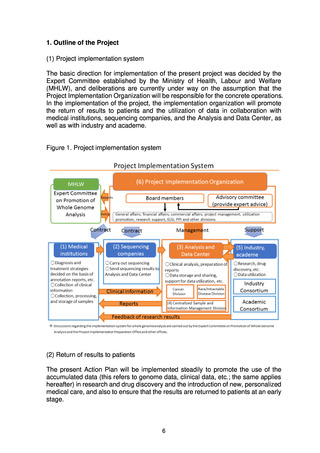
(1) Project implementation system .......................................................................................... 6
(2) Return of results to patients ............................................................................................... 6
(3) The type of medical care that whole genome analysis promotion aims for ...................... 9
2. Objectives of the Project ...................................................................................................... 9
3. Basic Strategies .................................................................................................................. 11
(1) Basic strategies for using the results of whole genome analysis in research and drug
discovery................................................................................................................................ 11
(2) Basic strategies for early introduction of analysis results intoclinical practice ................ 11
(3) Basic strategies to achieve new personalized medical care ........................................... 11
4. Initiatives to Date ................................................................................................................ 15
5. Basic Policy Based on Initiatives to Date ........................................................................ 17
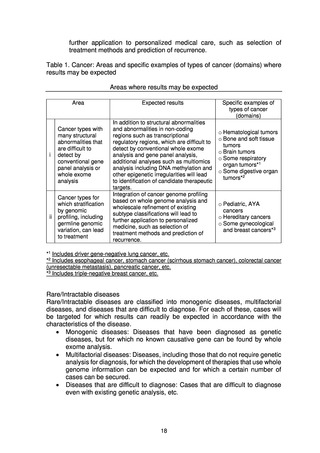
(1) Target patients for whole genome analysis ..................................................................... 17
(2) Numbers of target cases .................................................................................................. 19
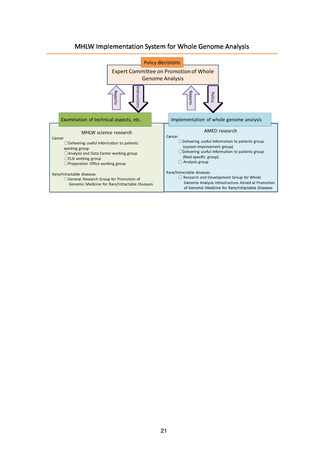
(3) MHLW implementation system for whole genome analysis ............................................ 19
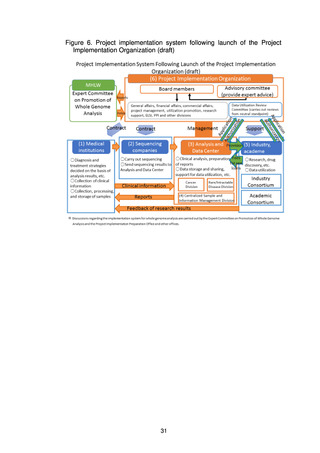
(4) Organizations that make up the project ........................................................................... 22
6. Policy and Details of Project Operations ......................................................................... 32
(1) Return of results to patients ............................................................................................. 32
(2) Utilization.......................................................................................................................... 45
(3) Development of human resources ................................................................................... 49
7. Items Relating to Ethical, Legal, and Social Issues (ELSI) ............................................ 51
8. Items Relating to Patient and Public Involvement (PPI) ................................................. 52
9. Conclusion ........................................................................................................................... 53
10. Glossary* ........................................................................................................................... 54
1
0. Foreword ................................................................................................................................ 2
1. Outline of the Project ............................................................................................................ 6
(1) Project implementation system .......................................................................................... 6
(2) Return of results to patients ............................................................................................... 6
(3) The type of medical care that whole genome analysis promotion aims for ...................... 9
2. Objectives of the Project ...................................................................................................... 9
3. Basic Strategies .................................................................................................................. 11
(1) Basic strategies for using the results of whole genome analysis in research and drug
discovery................................................................................................................................ 11
(2) Basic strategies for early introduction of analysis results intoclinical practice ................ 11
(3) Basic strategies to achieve new personalized medical care ........................................... 11
4. Initiatives to Date ................................................................................................................ 15
5. Basic Policy Based on Initiatives to Date ........................................................................ 17
(1) Target patients for whole genome analysis ..................................................................... 17
(2) Numbers of target cases .................................................................................................. 19
(3) MHLW implementation system for whole genome analysis ............................................ 19
(4) Organizations that make up the project ........................................................................... 22
6. Policy and Details of Project Operations ......................................................................... 32
(1) Return of results to patients ............................................................................................. 32
(2) Utilization.......................................................................................................................... 45
(3) Development of human resources ................................................................................... 49
7. Items Relating to Ethical, Legal, and Social Issues (ELSI) ............................................ 51
8. Items Relating to Patient and Public Involvement (PPI) ................................................. 52
9. Conclusion ........................................................................................................................... 53
10. Glossary* ........................................................................................................................... 54
1