よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (23 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
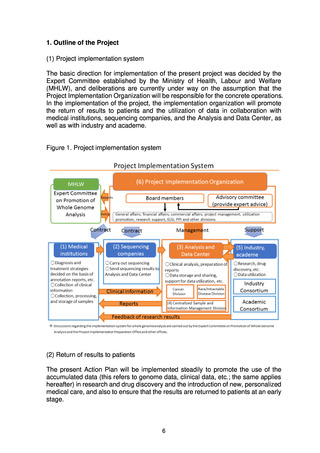
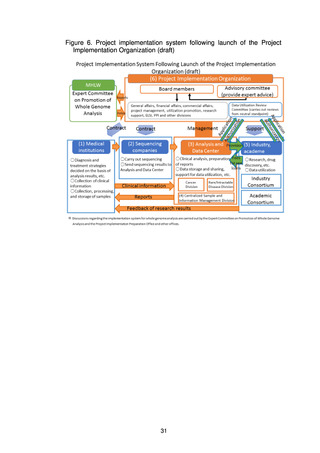
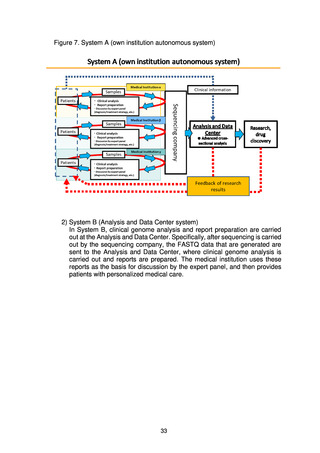
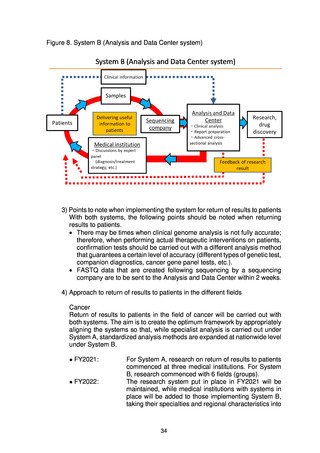
(4) Organizations that make up the project
(a) Medical institutions that return results to patients
To return the results of whole genome analysis to patients in an appropriate
fashion, medical institutions that return the results to patients are required to
have expert whole genome analysis personnel, a system for storage and
management of samples, ELSI compliance, advanced medical functions, and
systems for conducting clinical studies and trials.
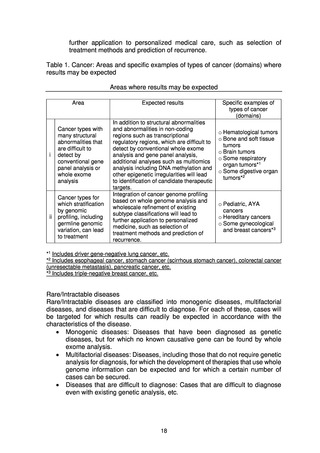
Cancer
The main requirements expected of medical institutions that return results to
patients are as follows.
The Expert Committee will examine and approve new medical institutions
meeting the requirements once a year, and institutions that are approved will be
added to the list of medical institutions that return results to patients the following
fiscal year. In addition, the Expert Committee will evaluate the systems and
performance of medical institutions that return results to patients once a year.
The Expert Committee will review the main requirements for medical institutions
that return results to patients as necessary.
o Main requirements for medical institutions that return results to patients
• The institution is a designated core hospital for cancer genomic medicine
or a designated hospital for cancer genomic medicine.
• The institution has medical staff engaged in cancer genomic medicine and
has treatment systems that allow the return of results to patients.
• Medical staff engaged in cancer genomic medicine receive mandatory
training that includes whole genome analysis to improve their genomic
literacy.
• There are systems in place that can evaluate the analytical validity and
clinical usefulness of the results of whole genome analysis. Specifically,
there are multiple genome researchers who are proficient in the following
items engaged in the project.
Confirmation of analysis data (including original data) and detection of
different types of call error
Interpretation of genomic alterations and attaching clinical significance
Verification of genomic alterations by confirmation tests with a certain
level of accuracy
• There are systems in place for the appropriate storage and management of
samples with the consent of patients.
• The institution has an appropriate system in place for conducting clinical
studies and trials, either on its own or in collaboration with other institutions,
and has a certain level of results.
Rare/Intractable diseases
In FY2021, the Verification Project for Whole Genome Analysis of rare/Intractable
Diseases (tentative name) carried out verification of methods relating to return of
results to patients, with the cooperation of five medical institutions that have experts
in the genome of rare/intractable diseases and are able to play a central role in
22
(a) Medical institutions that return results to patients
To return the results of whole genome analysis to patients in an appropriate
fashion, medical institutions that return the results to patients are required to
have expert whole genome analysis personnel, a system for storage and
management of samples, ELSI compliance, advanced medical functions, and
systems for conducting clinical studies and trials.
Cancer
The main requirements expected of medical institutions that return results to
patients are as follows.
The Expert Committee will examine and approve new medical institutions
meeting the requirements once a year, and institutions that are approved will be
added to the list of medical institutions that return results to patients the following
fiscal year. In addition, the Expert Committee will evaluate the systems and
performance of medical institutions that return results to patients once a year.
The Expert Committee will review the main requirements for medical institutions
that return results to patients as necessary.
o Main requirements for medical institutions that return results to patients
• The institution is a designated core hospital for cancer genomic medicine
or a designated hospital for cancer genomic medicine.
• The institution has medical staff engaged in cancer genomic medicine and
has treatment systems that allow the return of results to patients.
• Medical staff engaged in cancer genomic medicine receive mandatory
training that includes whole genome analysis to improve their genomic
literacy.
• There are systems in place that can evaluate the analytical validity and
clinical usefulness of the results of whole genome analysis. Specifically,
there are multiple genome researchers who are proficient in the following
items engaged in the project.
Confirmation of analysis data (including original data) and detection of
different types of call error
Interpretation of genomic alterations and attaching clinical significance
Verification of genomic alterations by confirmation tests with a certain
level of accuracy
• There are systems in place for the appropriate storage and management of
samples with the consent of patients.
• The institution has an appropriate system in place for conducting clinical
studies and trials, either on its own or in collaboration with other institutions,
and has a certain level of results.
Rare/Intractable diseases
In FY2021, the Verification Project for Whole Genome Analysis of rare/Intractable
Diseases (tentative name) carried out verification of methods relating to return of
results to patients, with the cooperation of five medical institutions that have experts
in the genome of rare/intractable diseases and are able to play a central role in
22