よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (17 ページ)
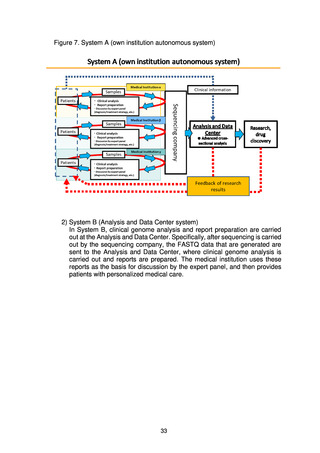
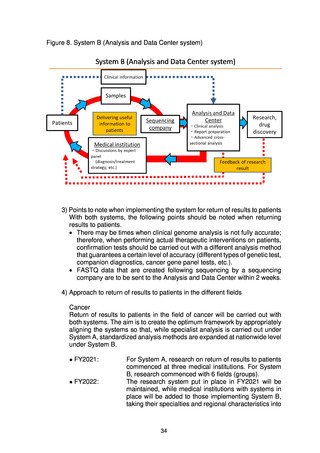
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
compiled in “Study for Promotion of the Action Plan for Whole Genome Analysis
(tentative name)” (February 2021) and “System Development for Further Promotion
of Whole Genome Analysis (tentative name)” (March 2021), respectively.
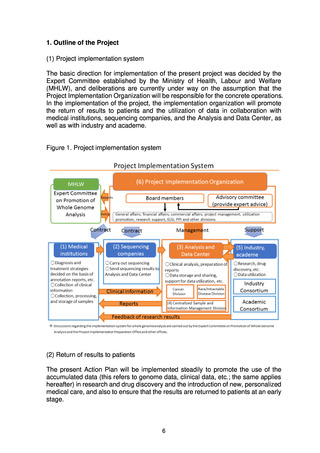
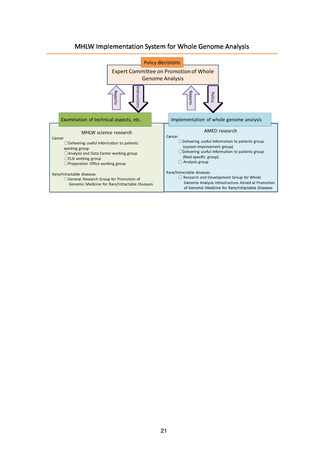
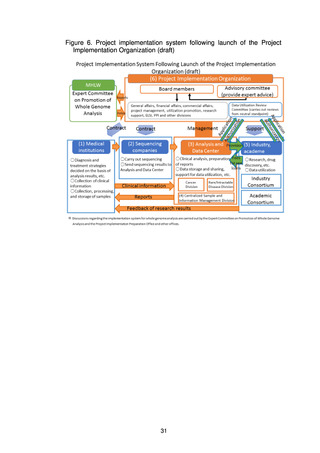
Since FY2021, the Expert Committee has been set up under the Science and
Technology Division of the MHLW’s Health Sciences Council to formulate the
Roadmap 2021 and to discuss the establishment of systems for return of results to
patients, the operation of the Analysis and Data Center, measures for utilization of
the data, measures for storage and utilization of samples, the operation of the
Project Implementation Organization, and the review system of the MHLW. At the
same time, whole genome analysis of approximately 10,000 samples, comprising
both stored and new samples, has been carried out.
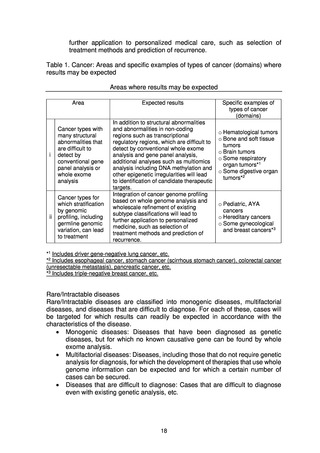
Rare/Intractable diseases
In the field of rare/intractable diseases, samples were selected for use in research
that met the conditions, such as whether the patient’s consent had been obtained
for the use of analysis results, whether the stored samples were of sufficient quality
for analysis, and whether clinical information was available. Taking into account the
opinion of an advisory council, the samples were classified into monogenic diseases,
multifactorial diseases, and difficult-to-diagnose diseases, and to the extent
possible with the current human resources and facilities, whole genome analysis
was conducted for diseases for which results could be expected (16approx. 2,500
cases in FY2020 and 3,000 cases in FY2021).
It was found that, whereas some patients were diagnosed with rare/intractable
diseases on the basis of clinical findings or existing genetic tests, among the
patients who were unable to receive a diagnosis through these methods, there were
some whose disease was identified as a result of the whole exome analysis in the
research.
In addition, it was shown that, in 9.4% of the patients whose disease was not
identified by whole exome analysis, the disease was identified as a result of whole
genome analysis.4
With whole exome analysis and whole genome analysis for the diagnosis of
patients who were not diagnosed by conventional methods, it is important to
determine the scope of applicable subjects and the methods of analysis, and further
research is needed for patients whose diseases are not identified by these analyses.
Based on these initiatives, the basic policy for whole genome analysis from FY2022
onward is summarized in the following chapter.
4 Practical Research Project for Rare/Intractable Diseases Research Group (Research
representative: Norihiro Kokudo, National Center for Global Health and Medicine)
16
(tentative name)” (February 2021) and “System Development for Further Promotion
of Whole Genome Analysis (tentative name)” (March 2021), respectively.
Since FY2021, the Expert Committee has been set up under the Science and
Technology Division of the MHLW’s Health Sciences Council to formulate the
Roadmap 2021 and to discuss the establishment of systems for return of results to
patients, the operation of the Analysis and Data Center, measures for utilization of
the data, measures for storage and utilization of samples, the operation of the
Project Implementation Organization, and the review system of the MHLW. At the
same time, whole genome analysis of approximately 10,000 samples, comprising
both stored and new samples, has been carried out.
Rare/Intractable diseases
In the field of rare/intractable diseases, samples were selected for use in research
that met the conditions, such as whether the patient’s consent had been obtained
for the use of analysis results, whether the stored samples were of sufficient quality
for analysis, and whether clinical information was available. Taking into account the
opinion of an advisory council, the samples were classified into monogenic diseases,
multifactorial diseases, and difficult-to-diagnose diseases, and to the extent
possible with the current human resources and facilities, whole genome analysis
was conducted for diseases for which results could be expected (16approx. 2,500
cases in FY2020 and 3,000 cases in FY2021).
It was found that, whereas some patients were diagnosed with rare/intractable
diseases on the basis of clinical findings or existing genetic tests, among the
patients who were unable to receive a diagnosis through these methods, there were
some whose disease was identified as a result of the whole exome analysis in the
research.
In addition, it was shown that, in 9.4% of the patients whose disease was not
identified by whole exome analysis, the disease was identified as a result of whole
genome analysis.4
With whole exome analysis and whole genome analysis for the diagnosis of
patients who were not diagnosed by conventional methods, it is important to
determine the scope of applicable subjects and the methods of analysis, and further
research is needed for patients whose diseases are not identified by these analyses.
Based on these initiatives, the basic policy for whole genome analysis from FY2022
onward is summarized in the following chapter.
4 Practical Research Project for Rare/Intractable Diseases Research Group (Research
representative: Norihiro Kokudo, National Center for Global Health and Medicine)
16