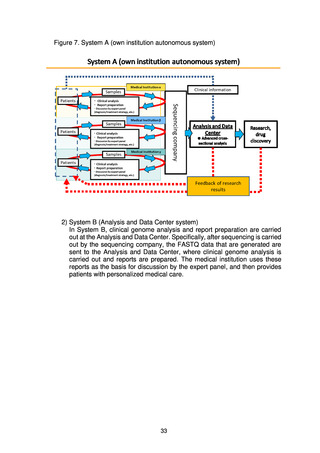
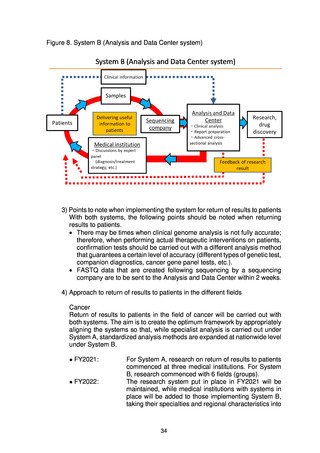
よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (43 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
Rather than being limited to a particular electronic record vendor,
prototyping of programs to convert diverse electronic record data held
by multiple medical institutions to a standard format will be carried out.
Push type and pull type systems for sending and receiving clinical
information will be compared, and issues or areas for improvement
will be verified through creation of a prototype.
The security conditions for data management and system construction
will be met.
• Clinical information database
The clinical information collected by the Analysis and Data Center will
use a cloud service. A prototype system will be constructed in FY2022,
and any issues or points for improvement will be verified, with the aim of
implementation from FY2023 onward. In this regard, the following points
should be noted.
For the method of description within the clinical database structure,
multiple methods will be compared, and one that meets the
performance requirements will be selected.
For the unstructured database, advanced methods will be compared
and verified, taking into consideration the patterns of utilization.
The database will be of a type that can ensure user-friendliness for
the expert panel, as well as search and other functional capabilities.
The database will satisfy the security requirements for data
management and system construction.
The clinical information items that are collected will need to
encompass the information required for various applications, including
care of the corresponding patient, clinical research, and drug
discovery. Items common to cancer and rare/intractable diseases will
be collected, as well as additional items of information that depend on
the characteristics of the disease.9
The system will be capable of adding additional items of clinical
information as necessary.
Unified pipeline
A unified pipeline will be constructed to centrally and collectively perform
the common parts of the analysis process carried out by many
researchers. Commonly used analysis tools and parameters will be
selected, based on domestic and international trends, to ensure future
data sharing and compatibility with various databases. In addition,
periodic reviews will be conducted.
For the cloud-based genome analysis system infrastructure, multiple
services will be selected from the cloud services that can meet the
performance, availability, security, and scalability requirements of the
unified pipeline. These will be compared in terms of performance and
cost, any other issues will be identified, and a prototype will be built
during FY2022. The configuration will be upgraded in stages from
9 Table of Items of Clinical Information Collected in Relation to Cancer Whole Genome
https://www.mhlw.go.jp/content/10901000/000833423.pdf
42
prototyping of programs to convert diverse electronic record data held
by multiple medical institutions to a standard format will be carried out.
Push type and pull type systems for sending and receiving clinical
information will be compared, and issues or areas for improvement
will be verified through creation of a prototype.
The security conditions for data management and system construction
will be met.
• Clinical information database
The clinical information collected by the Analysis and Data Center will
use a cloud service. A prototype system will be constructed in FY2022,
and any issues or points for improvement will be verified, with the aim of
implementation from FY2023 onward. In this regard, the following points
should be noted.
For the method of description within the clinical database structure,
multiple methods will be compared, and one that meets the
performance requirements will be selected.
For the unstructured database, advanced methods will be compared
and verified, taking into consideration the patterns of utilization.
The database will be of a type that can ensure user-friendliness for
the expert panel, as well as search and other functional capabilities.
The database will satisfy the security requirements for data
management and system construction.
The clinical information items that are collected will need to
encompass the information required for various applications, including
care of the corresponding patient, clinical research, and drug
discovery. Items common to cancer and rare/intractable diseases will
be collected, as well as additional items of information that depend on
the characteristics of the disease.9
The system will be capable of adding additional items of clinical
information as necessary.
Unified pipeline
A unified pipeline will be constructed to centrally and collectively perform
the common parts of the analysis process carried out by many
researchers. Commonly used analysis tools and parameters will be
selected, based on domestic and international trends, to ensure future
data sharing and compatibility with various databases. In addition,
periodic reviews will be conducted.
For the cloud-based genome analysis system infrastructure, multiple
services will be selected from the cloud services that can meet the
performance, availability, security, and scalability requirements of the
unified pipeline. These will be compared in terms of performance and
cost, any other issues will be identified, and a prototype will be built
during FY2022. The configuration will be upgraded in stages from
9 Table of Items of Clinical Information Collected in Relation to Cancer Whole Genome
https://www.mhlw.go.jp/content/10901000/000833423.pdf
42