よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (12 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
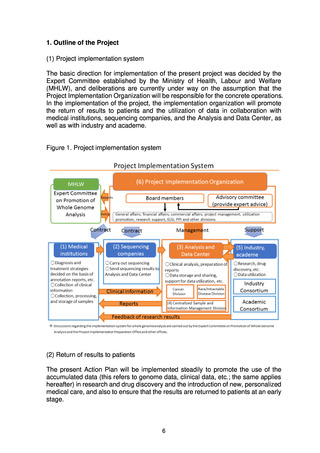
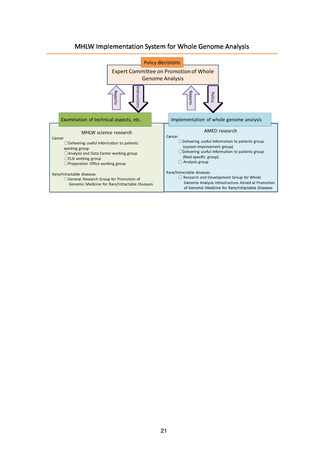
3. Basic Strategies
The basic strategies for achieving the objectives of the project have been
determined, and practical application will start in fields where results are obtained,
with the aim of overcoming cancer and rare/intractable diseases in the future.
Promoting the strategic accumulation of data and facilitating research and drug
discovery that use these data will be essential for achieving this aim, and the basic
strategies for using the results of whole genome analysis in research and drug
discovery have therefore been drawn up as follows.
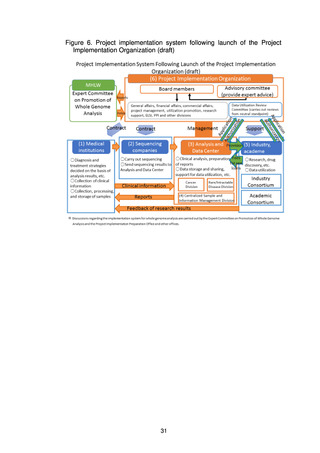
(1) Basic strategies for using the results of whole genome analysis in research
and drug discovery
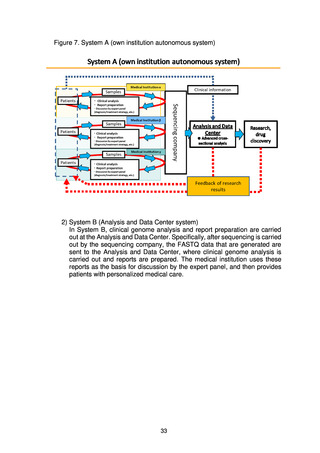
To ensure comprehensive return of the results of whole genome analysis to
patients, it is important to put in place an environment that stimulates research
and drug discovery using the accumulated genome data. Initiatives in
collaboration with industry and academe will therefore be promoted with the aim
of nurturing industries through the creation of innovation originating in Japan,
while at the same time new treatments will be made available to patients.
To enable this, a system is needed to allow open and fair use of the
accumulated whole genome analysis information by researchers in domestic and
overseas research institutions and companies. An Industry Consortium and an
Academic Consortium will therefore be established, and the Project
Implementation Organization will create a mechanism to support their
collaboration.
In addition, the basic strategies for early introduction of analysis results into
clinical practice and for implementing new personalized medical care are as
follows.
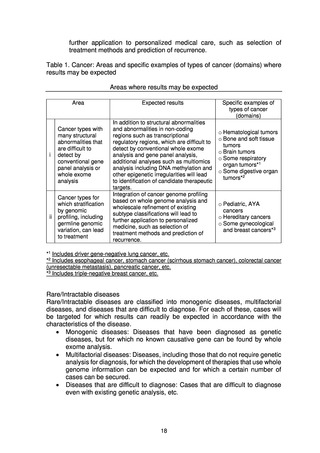
(2) Basic strategies for early introduction of analysis results into clinical practice
Where the results of whole genome analysis relate to diagnosis or treatment with
efficacy that has already been confirmed, existing drugs, etc. will be made
available promptly to patients through clinical research.
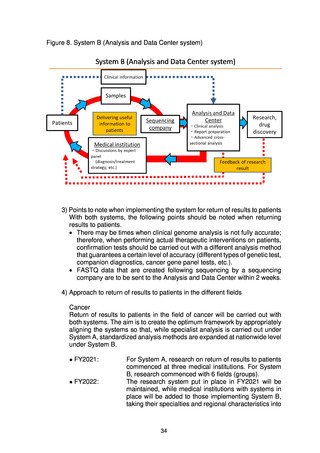
In addition, when the results of whole genome analysis are returned to
patients, confirmation tests with a certain level of accuracy will be performed, and
systems for this will be put in place within medical institutions. Where the results
of genome analysis give a certain degree of new evidence for technologies to
select appropriate treatment methods or diagnose diseases, the goal will be for
them to be covered by national health insurance in the future.
These measures are expected to make it possible to provide patients with
appropriate treatment at an early stage, thus contributing to enhanced treatment
opportunities and more efficient provision of medical care.
(3) Basic strategies to achieve new personalized medical care
New clinical studies and trials will be conducted, in addition to which real-world
evidence will be compiled to achieve more personalized medical care through
advanced, efficient diagnosis and treatment based on whole genome analysis
and multiomics analysis, etc. Efficient clinical studies and trials will also be
promoted through the use of clinical study support tools.
11
The basic strategies for achieving the objectives of the project have been
determined, and practical application will start in fields where results are obtained,
with the aim of overcoming cancer and rare/intractable diseases in the future.
Promoting the strategic accumulation of data and facilitating research and drug
discovery that use these data will be essential for achieving this aim, and the basic
strategies for using the results of whole genome analysis in research and drug
discovery have therefore been drawn up as follows.
(1) Basic strategies for using the results of whole genome analysis in research
and drug discovery
To ensure comprehensive return of the results of whole genome analysis to
patients, it is important to put in place an environment that stimulates research
and drug discovery using the accumulated genome data. Initiatives in
collaboration with industry and academe will therefore be promoted with the aim
of nurturing industries through the creation of innovation originating in Japan,
while at the same time new treatments will be made available to patients.
To enable this, a system is needed to allow open and fair use of the
accumulated whole genome analysis information by researchers in domestic and
overseas research institutions and companies. An Industry Consortium and an
Academic Consortium will therefore be established, and the Project
Implementation Organization will create a mechanism to support their
collaboration.
In addition, the basic strategies for early introduction of analysis results into
clinical practice and for implementing new personalized medical care are as
follows.
(2) Basic strategies for early introduction of analysis results into clinical practice
Where the results of whole genome analysis relate to diagnosis or treatment with
efficacy that has already been confirmed, existing drugs, etc. will be made
available promptly to patients through clinical research.
In addition, when the results of whole genome analysis are returned to
patients, confirmation tests with a certain level of accuracy will be performed, and
systems for this will be put in place within medical institutions. Where the results
of genome analysis give a certain degree of new evidence for technologies to
select appropriate treatment methods or diagnose diseases, the goal will be for
them to be covered by national health insurance in the future.
These measures are expected to make it possible to provide patients with
appropriate treatment at an early stage, thus contributing to enhanced treatment
opportunities and more efficient provision of medical care.
(3) Basic strategies to achieve new personalized medical care
New clinical studies and trials will be conducted, in addition to which real-world
evidence will be compiled to achieve more personalized medical care through
advanced, efficient diagnosis and treatment based on whole genome analysis
and multiomics analysis, etc. Efficient clinical studies and trials will also be
promoted through the use of clinical study support tools.
11