よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (52 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
7. Items Relating to Ethical, Legal, and Social Issues (ELSI)
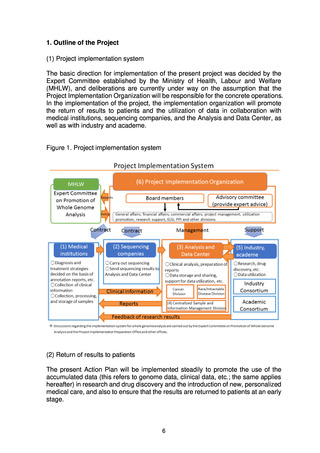
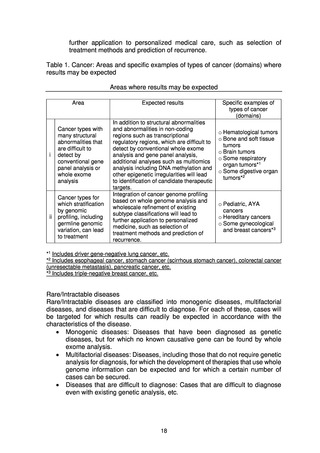
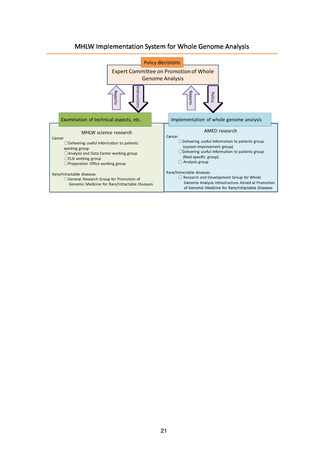
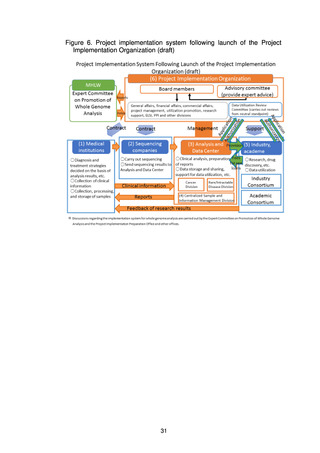
The present Action Plan stipulates the implementation of whole genome analysis
on an unprecedented scale in Japan, and it includes database construction,
facilitation of research and development for drug discovery and diagnostic
technologies, and return of the results of whole genome analysis to patients.
Implementation of these projects may be expected to involve various ethical, legal,
and social issues (ELSI). For the present project to be properly implemented based
on the understanding and trust of society, it will be essential to appropriately
address ELSI and to put in place systems to achieve this.
Specifically, an ELSI Division staffed by personnel with expertise in this field
will be set up within the Project Implementation Organization, and this division will
examine and take action on initiatives needed to ensure that the plan for the project
overall is being implemented with due regard for ELSI.
The following points in particular should be noted with regard to taking action over
ELSI.
• A standardized ICF will be used to enable cross-sectional data utilization.
• The use of ICT and AI in clinical implementation of whole genome analysis
should be examined from the perspective of reducing the workload in clinical
settings.
• The guidance prepared by the MHLW research group will be used to explain
the project to patients, and explanations and information will be provided as
clearly and carefully as possible to respect the free will of patients and seek
their informed consent. The necessary systems, including the use of econsent, will be considered.
• Opportunities to receive genetic counseling will be ensured and enhanced,
which will include the active use of ICT.
• If findings other than the main findings are obtained, the ethical guidelines and
the guidance prepared by the MHLW research group will be referred to when
taking action.
• The policy on information security and protection of privacy will be clarified,
the necessary systems for implementation will be established, and patients
will be informed about these systems.
• Institutional issues in the development of a social environment to ensure there
are no disadvantages resulting from genome information will be identified, and
the policy of the present project for addressing these issues will be examined.
• The consultation system for whole genome analysis will be improved, and the
development and expansion of support systems will be promoted through
education and awareness activities so that existing consultation organizations
can provide primary consultation services.
51
The present Action Plan stipulates the implementation of whole genome analysis
on an unprecedented scale in Japan, and it includes database construction,
facilitation of research and development for drug discovery and diagnostic
technologies, and return of the results of whole genome analysis to patients.
Implementation of these projects may be expected to involve various ethical, legal,
and social issues (ELSI). For the present project to be properly implemented based
on the understanding and trust of society, it will be essential to appropriately
address ELSI and to put in place systems to achieve this.
Specifically, an ELSI Division staffed by personnel with expertise in this field
will be set up within the Project Implementation Organization, and this division will
examine and take action on initiatives needed to ensure that the plan for the project
overall is being implemented with due regard for ELSI.
The following points in particular should be noted with regard to taking action over
ELSI.
• A standardized ICF will be used to enable cross-sectional data utilization.
• The use of ICT and AI in clinical implementation of whole genome analysis
should be examined from the perspective of reducing the workload in clinical
settings.
• The guidance prepared by the MHLW research group will be used to explain
the project to patients, and explanations and information will be provided as
clearly and carefully as possible to respect the free will of patients and seek
their informed consent. The necessary systems, including the use of econsent, will be considered.
• Opportunities to receive genetic counseling will be ensured and enhanced,
which will include the active use of ICT.
• If findings other than the main findings are obtained, the ethical guidelines and
the guidance prepared by the MHLW research group will be referred to when
taking action.
• The policy on information security and protection of privacy will be clarified,
the necessary systems for implementation will be established, and patients
will be informed about these systems.
• Institutional issues in the development of a social environment to ensure there
are no disadvantages resulting from genome information will be identified, and
the policy of the present project for addressing these issues will be examined.
• The consultation system for whole genome analysis will be improved, and the
development and expansion of support systems will be promoted through
education and awareness activities so that existing consultation organizations
can provide primary consultation services.
51