よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (29 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
facilitate research and development of drug discovery and diagnostic
technologies and deliver the results to patients promptly.
1) The role of industry
The aim is to develop new diagnostic technologies and therapeutic drugs
based on data obtained from whole genome analysis. For this, an Industry
Consortium hosted by industrial circles will be established to enable industry
to participate proactively in the project, including the data collection process,
from the very beginning.
The main objective of the Industry Consortium will be to promote
research and development of drug discovery and diagnostic technologies
using whole genome analysis data. The consortium will be organized by the
pharmaceutical and other industries as an organization in which many
different medical and non-medical industries, as well as venture capital
companies, can participate, and the aim is to launch it during FY2022. In
addition, specific operating rules that will include setting incentives to
utilization the data according to personnel, technological, and financial
cooperation from each company will be decided.
2) The role of academe
The aim is to promote research into genomic medicine on the basis of the
data obtained from whole genome analysis. For this, an Academic
Consortium hosted by the academic world will be established as a
nationwide Japanese scholarly institution to hold proactive academic
discussions regarding whole genome analysis.
The Academic Consortium will be expected, in return for sharing data
relating to whole genome analysis and being granted the authority to make
widespread utilization of the data, to play the roles of establishing groups of
experts in different fields, holding discussions on the clinical and pathological
significance of findings on new mutations identified as a result of advanced
cross-sectional analyses, and after accumulation of a range of necessary
data, giving judgments by expert panels on whether the data merit being
returned to patients.
Specific operational rules for the Academic Consortium, such as
reviewing applications for membership on an organization-by-organization
basis, registration of affiliated researchers, and coordination of joint research,
will be established with the aim of launching the consortium in FY2022.
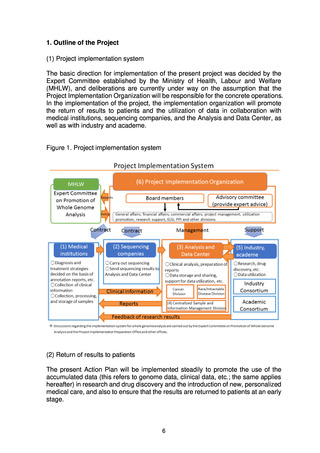
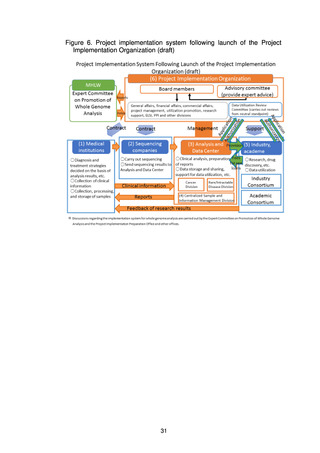
3) Support for industry and academe by the Project Implementation
Organization
The Project Implementation Organization will provide operational support to
the Industry Consortium and the Academic Consortium to facilitate
mechanisms for the prompt return of new knowledge to the general public.
Specifically, the Project Implementation Organization will set up a
division to provide the Industry Consortium with operational support for
further promotion of development projects using the data. This division will
give support for data utilization, intellectual property management, proposals
for new research, collaboration with the Academic Consortium, and matching
companies for collaboration.
28
technologies and deliver the results to patients promptly.
1) The role of industry
The aim is to develop new diagnostic technologies and therapeutic drugs
based on data obtained from whole genome analysis. For this, an Industry
Consortium hosted by industrial circles will be established to enable industry
to participate proactively in the project, including the data collection process,
from the very beginning.
The main objective of the Industry Consortium will be to promote
research and development of drug discovery and diagnostic technologies
using whole genome analysis data. The consortium will be organized by the
pharmaceutical and other industries as an organization in which many
different medical and non-medical industries, as well as venture capital
companies, can participate, and the aim is to launch it during FY2022. In
addition, specific operating rules that will include setting incentives to
utilization the data according to personnel, technological, and financial
cooperation from each company will be decided.
2) The role of academe
The aim is to promote research into genomic medicine on the basis of the
data obtained from whole genome analysis. For this, an Academic
Consortium hosted by the academic world will be established as a
nationwide Japanese scholarly institution to hold proactive academic
discussions regarding whole genome analysis.
The Academic Consortium will be expected, in return for sharing data
relating to whole genome analysis and being granted the authority to make
widespread utilization of the data, to play the roles of establishing groups of
experts in different fields, holding discussions on the clinical and pathological
significance of findings on new mutations identified as a result of advanced
cross-sectional analyses, and after accumulation of a range of necessary
data, giving judgments by expert panels on whether the data merit being
returned to patients.
Specific operational rules for the Academic Consortium, such as
reviewing applications for membership on an organization-by-organization
basis, registration of affiliated researchers, and coordination of joint research,
will be established with the aim of launching the consortium in FY2022.
3) Support for industry and academe by the Project Implementation
Organization
The Project Implementation Organization will provide operational support to
the Industry Consortium and the Academic Consortium to facilitate
mechanisms for the prompt return of new knowledge to the general public.
Specifically, the Project Implementation Organization will set up a
division to provide the Industry Consortium with operational support for
further promotion of development projects using the data. This division will
give support for data utilization, intellectual property management, proposals
for new research, collaboration with the Academic Consortium, and matching
companies for collaboration.
28