よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (18 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
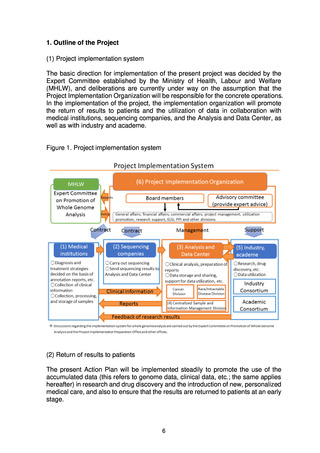
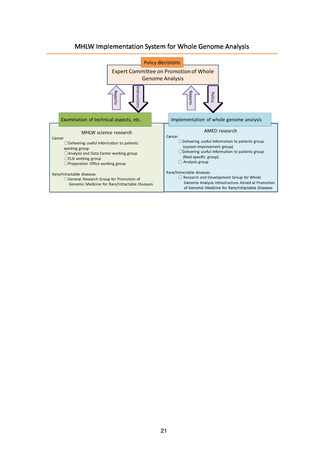
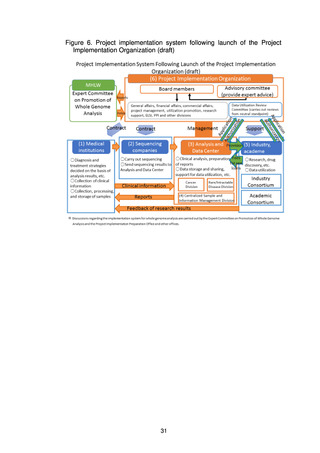
5. Basic Policy Based on Initiatives to Date
(1) Target patients for whole genome analysis
Based on the findings obtained from whole genome analysis to date, the target
patients for whole genome analysis are patients who are difficult to diagnose or
have a low probability of radical cure through existing medical care, but who may
be expected to obtain more accurate diagnosis and effective treatment through the
use of whole genome analysis or multiomics analysis. Specifically, the target
patients are as shown below.
Cancer
A. Target patients
As a general rule, the targets for analysis will be patients who fulfill all of the
following three conditions and who give new consent following a full explanation.
(a) Sufficient samples can be obtained through surgery, biopsy, blood
sampling (hematological tumors), etc.
(b) The patient has refractory cancer with a low probability of radical cure
through surgery (unresectable advanced cancer, cancer with high
probability of recurrence, etc.).
(c) The patient is alive at the start of the analysis and may be expected to
receive some form of treatment in the future.
Other patients may be targets for analysis only in the event that the Expert
Committee gives its approval in view of the importance of the disease.*
* In the search for markers for future drug development based on the genome
database of cancers in Japanese people, important diseases are expected to
include rare cancers, adolescent and young adult (AYA) cancers, pediatric cancers,
hereditary cancers, treatment-resistant and refractory cancers, cancers with a small
number of cases but that are characteristically Japanese (such as adult T-cell
leukemia), and cancers with a large number of cases but for which genome
information on Japanese cases has not been sufficiently accumulated.
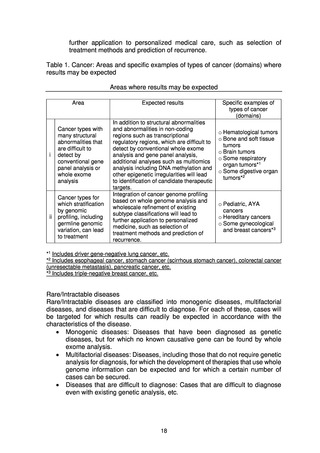
B. Fields in which results may be expected
From among the target patients, the following two fields are expected to yield
results.
(a) Cancer types with many structural abnormalities that are difficult to detect
by conventional gene panel analysis or whole exome analysis
In addition to structural abnormalities and abnormalities in non-coding
regions such as transcriptional regulatory regions, which are difficult to
detect by conventional whole exome analysis and gene panel analysis,
additional analyses such as multiomics analysis including DNA methylation
and other epigenetic irregularities will lead to identification of candidate
therapeutic targets.
(b) Cancer types for which stratification by genomic profiling, including
germline genomic variation, can lead to treatment
Integration of cancer genome profiling based on whole genome analysis
and wholescale refinement of existing subtype classifications will lead to
17
(1) Target patients for whole genome analysis
Based on the findings obtained from whole genome analysis to date, the target
patients for whole genome analysis are patients who are difficult to diagnose or
have a low probability of radical cure through existing medical care, but who may
be expected to obtain more accurate diagnosis and effective treatment through the
use of whole genome analysis or multiomics analysis. Specifically, the target
patients are as shown below.
Cancer
A. Target patients
As a general rule, the targets for analysis will be patients who fulfill all of the
following three conditions and who give new consent following a full explanation.
(a) Sufficient samples can be obtained through surgery, biopsy, blood
sampling (hematological tumors), etc.
(b) The patient has refractory cancer with a low probability of radical cure
through surgery (unresectable advanced cancer, cancer with high
probability of recurrence, etc.).
(c) The patient is alive at the start of the analysis and may be expected to
receive some form of treatment in the future.
Other patients may be targets for analysis only in the event that the Expert
Committee gives its approval in view of the importance of the disease.*
* In the search for markers for future drug development based on the genome
database of cancers in Japanese people, important diseases are expected to
include rare cancers, adolescent and young adult (AYA) cancers, pediatric cancers,
hereditary cancers, treatment-resistant and refractory cancers, cancers with a small
number of cases but that are characteristically Japanese (such as adult T-cell
leukemia), and cancers with a large number of cases but for which genome
information on Japanese cases has not been sufficiently accumulated.
B. Fields in which results may be expected
From among the target patients, the following two fields are expected to yield
results.
(a) Cancer types with many structural abnormalities that are difficult to detect
by conventional gene panel analysis or whole exome analysis
In addition to structural abnormalities and abnormalities in non-coding
regions such as transcriptional regulatory regions, which are difficult to
detect by conventional whole exome analysis and gene panel analysis,
additional analyses such as multiomics analysis including DNA methylation
and other epigenetic irregularities will lead to identification of candidate
therapeutic targets.
(b) Cancer types for which stratification by genomic profiling, including
germline genomic variation, can lead to treatment
Integration of cancer genome profiling based on whole genome analysis
and wholescale refinement of existing subtype classifications will lead to
17