よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (20 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_35569.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第17回 10/3)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
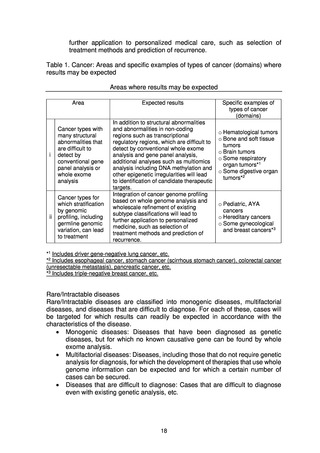
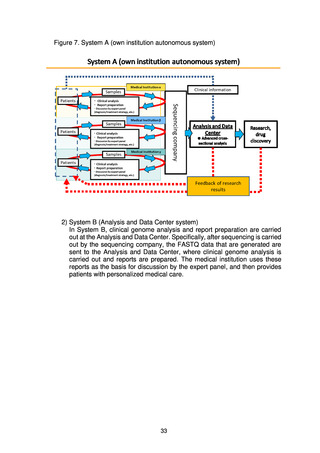
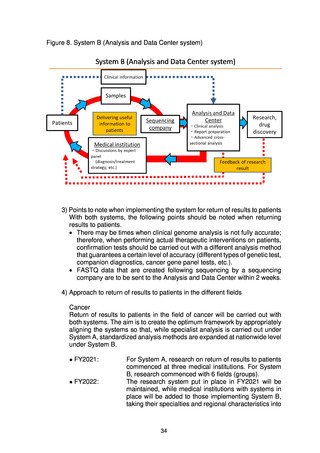
(2) Numbers of target cases
Whole genome analysis of approximately 19,200 cases (cancer: approx. 13,700,
rare/intractable diseases: approx. 5,500) was carried out from 2019 to FY2021. In
FY2022, the plan is to analyze 4,500 cases (cancer: approx. 2,000, rare/intractable
diseases: approx. 2,500) of new patients giving consent and to return the results to
patients.*
* As well as the results from the analysis targeting 100,000 genomes, this includes
the results of multiomics analyses (comprehensive information on biomolecules),
etc.
Cancer
In FY2021, whole genome analysis of around 600 prospective new patients was carried out at
three medical institutions. From FY2022 onward, analyses will be carried out at medical
institutions that are recognized by the Expert Committee as having the necessary systems in
place for appropriate return of the results of whole genome analysis to patients, from among
the 12 designated core hospitals for cancer genomic medicine and 33 designated hospitals
for cancer genomic medicine (tentative name)(as of January 2022). In addition, from
FY2023 onward, systems will be put in place in a stepwise fashion that will include examining
the participation of medical institutions that can ensure a cancer genomic medicine treatment
system, through collaboration with medical institutions approved by the Expert Committee.
In the field of cancer, analysis of approximately 2,000 cases will be carried out
in FY2022 on the basis of the situation up to FY2021. Specific figures for the number
of analyses from FY2023 onward will be considered on the basis of the situation to
date, including any changes in the number of patients being examined.
Rare/Intractable diseases
In the field of rare/intractable diseases, analysis of approximately 2,500 cases will
be carried out in FY2022 on the basis of the situation up to FY2021. As with the
field of cancer, specific figures for the number of analyses from FY2023 onward will
be considered on the basis of the situation to date.
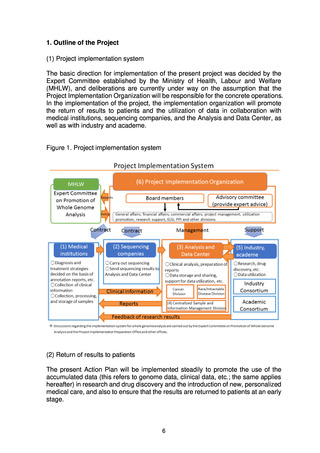
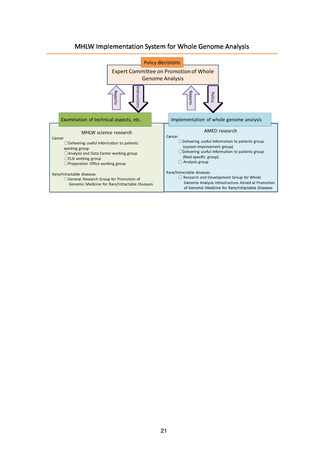
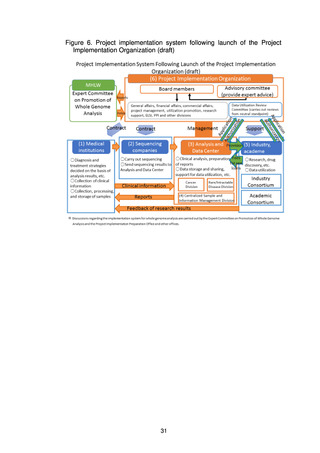
(3) MHLW implementation system for whole genome analysis
(a) Expert Committee on Promotion of Whole Genome Analysis
The Expert Committee, which was established under the Science and
Technology Division of the MHLW’s Health Sciences Council, is the top
decision-making body with regard to the promotion of whole genome analysis.
The Expert Committee will hold discussions aimed at steady promotion of the
present Action Plan and will check the progress of initiatives based on the
Action Plan and make necessary decisions. In addition, the Expert Committee
will hold consultations as necessary on review of the present Action Plan.
These measures will ensure a highly workable review framework for the
present project, in which the responsibility of the government is made evident.
The Expert Committee will continue to be the top decision-making body
for the government’s basic policy with regard to promotion of whole genome
analysis even after the launch of the Project Implementation Organization.
(b) MHLW science research group
The MHLW science research group comprises experts well versed in the
practical aspects of whole genome analysis, who will study the technical
19
Whole genome analysis of approximately 19,200 cases (cancer: approx. 13,700,
rare/intractable diseases: approx. 5,500) was carried out from 2019 to FY2021. In
FY2022, the plan is to analyze 4,500 cases (cancer: approx. 2,000, rare/intractable
diseases: approx. 2,500) of new patients giving consent and to return the results to
patients.*
* As well as the results from the analysis targeting 100,000 genomes, this includes
the results of multiomics analyses (comprehensive information on biomolecules),
etc.
Cancer
In FY2021, whole genome analysis of around 600 prospective new patients was carried out at
three medical institutions. From FY2022 onward, analyses will be carried out at medical
institutions that are recognized by the Expert Committee as having the necessary systems in
place for appropriate return of the results of whole genome analysis to patients, from among
the 12 designated core hospitals for cancer genomic medicine and 33 designated hospitals
for cancer genomic medicine (tentative name)(as of January 2022). In addition, from
FY2023 onward, systems will be put in place in a stepwise fashion that will include examining
the participation of medical institutions that can ensure a cancer genomic medicine treatment
system, through collaboration with medical institutions approved by the Expert Committee.
In the field of cancer, analysis of approximately 2,000 cases will be carried out
in FY2022 on the basis of the situation up to FY2021. Specific figures for the number
of analyses from FY2023 onward will be considered on the basis of the situation to
date, including any changes in the number of patients being examined.
Rare/Intractable diseases
In the field of rare/intractable diseases, analysis of approximately 2,500 cases will
be carried out in FY2022 on the basis of the situation up to FY2021. As with the
field of cancer, specific figures for the number of analyses from FY2023 onward will
be considered on the basis of the situation to date.
(3) MHLW implementation system for whole genome analysis
(a) Expert Committee on Promotion of Whole Genome Analysis
The Expert Committee, which was established under the Science and
Technology Division of the MHLW’s Health Sciences Council, is the top
decision-making body with regard to the promotion of whole genome analysis.
The Expert Committee will hold discussions aimed at steady promotion of the
present Action Plan and will check the progress of initiatives based on the
Action Plan and make necessary decisions. In addition, the Expert Committee
will hold consultations as necessary on review of the present Action Plan.
These measures will ensure a highly workable review framework for the
present project, in which the responsibility of the government is made evident.
The Expert Committee will continue to be the top decision-making body
for the government’s basic policy with regard to promotion of whole genome
analysis even after the launch of the Project Implementation Organization.
(b) MHLW science research group
The MHLW science research group comprises experts well versed in the
practical aspects of whole genome analysis, who will study the technical
19