よむ、つかう、まなぶ。
07 参考資料2-1 帯状疱疹ワクチン ファクトシート (54 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_36248.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会 予防接種基本方針部会 ワクチン評価に関する小委員会(第21回 11/9)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
134. Vermeulen JN, Lange JM, Tyring SK, Peters PH, Nunez M, Poland G, Levin MJ, Freeman
C, Chalikonda I, Li J, Smith JG, Caulfield MJ, Stek JE, Chan IS, Vessey R, Schödel FP,
Annunziato PW, Schlienger K, Silber JL. Safety, tolerability, and immunogenicity after 1
and 2 doses of zoster vaccine in healthy adults ≥60 years of age. Vaccine 2011; 30(5):
904-10.
135. Vesikari T, Hardt R, Rümke HC, Icardi G, Montero J, Thomas S, Sadorge C, Fiquet A.
Immunogenicity and safety of a live attenuated shingles (herpes zoster) vaccine (Zostavax)
in individuals aged ≥ 70 years. Hum Vaccin Immunother 2013; 9(4): 858-64.
136. Diez-Domingo J, Weinke T, Garcia de Lomas J, Meyer CU, Bertrand I, Eymin C, Thomas
S, Sadorge C. Comparison of intramuscular and subcutaneous administration of
a herpes zoster live-attenuatedvaccine in
adults
aged
≥50
years:
a
randomised
non-inferiority clinical trial. Vaccine 2015; 33: 789-95.
137. Kerzner B, Murray AV, Cheng E, Ifle R, Harvey PR, Tomlinson M, Barben JL, Rarrick K,
Stek JE, Chung MO, Schödel FP, Wang WW, Xu J, Chan IS, Silber JL, Schlienger K.
Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and
inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc 2007; 55(10):
1499-507.
138. MacIntyre CR, Egerton T, McCaughey M, Parrino J, Campbell BV, Su SC, Pagnoni MF,
Stek JE, Xu J, Annunziato PW, Chan IS, Silber JL. Concomitant administration of zoster
and pneumococcal vaccines in adults ≥60 years old. Hum Vaccines 2010; 6(11): 894-902.
139. Chlibek R, Pauksens K, Rombo L, van Rijckevorsel G, Richardus JH, Plassmann
G, Schwarz TF, Catteau G, Lal H, Heineman TC. Long-term immunogenicity and safety of
an investigational herpes zoster subunit vaccine in older adults. Vaccine 2016; 34: 863-8.
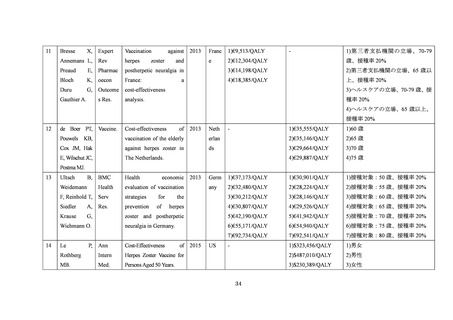
140. de Boer PT, Wilschut JC, Postma MJ. Cost-effectiveness of vaccination against herpes
zoster. Hum Vaccin Immunother 2014; 10(7): 2048-61.
141. Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of
vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine
2014; 32(15): 1645-53.
142. Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 Years.
Ann Intern Med 2015; 163(7): 489-97.
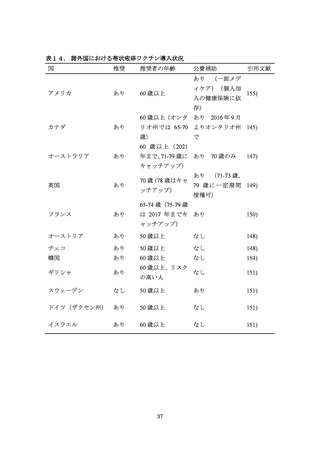
143. Advisory Committee on Immunization Practices (ACIP) Summary Report February 24-25,
2010. https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-feb10.pdf
144. Immunize Canada. Herpes Zoster (Shingles). Available:
http://www.immunize.ca/en/diseases-vaccines/herpeszoster.aspx
145. Ontario. Ontario Making Shingles Vaccine Free for Seniors. Available:
https://news.ontario.ca/mohltc/en/2016/09/ontario-making-shingles-vaccine-free-for-seniors.html
49
C, Chalikonda I, Li J, Smith JG, Caulfield MJ, Stek JE, Chan IS, Vessey R, Schödel FP,
Annunziato PW, Schlienger K, Silber JL. Safety, tolerability, and immunogenicity after 1
and 2 doses of zoster vaccine in healthy adults ≥60 years of age. Vaccine 2011; 30(5):
904-10.
135. Vesikari T, Hardt R, Rümke HC, Icardi G, Montero J, Thomas S, Sadorge C, Fiquet A.
Immunogenicity and safety of a live attenuated shingles (herpes zoster) vaccine (Zostavax)
in individuals aged ≥ 70 years. Hum Vaccin Immunother 2013; 9(4): 858-64.
136. Diez-Domingo J, Weinke T, Garcia de Lomas J, Meyer CU, Bertrand I, Eymin C, Thomas
S, Sadorge C. Comparison of intramuscular and subcutaneous administration of
a herpes zoster live-attenuatedvaccine in
adults
aged
≥50
years:
a
randomised
non-inferiority clinical trial. Vaccine 2015; 33: 789-95.
137. Kerzner B, Murray AV, Cheng E, Ifle R, Harvey PR, Tomlinson M, Barben JL, Rarrick K,
Stek JE, Chung MO, Schödel FP, Wang WW, Xu J, Chan IS, Silber JL, Schlienger K.
Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and
inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc 2007; 55(10):
1499-507.
138. MacIntyre CR, Egerton T, McCaughey M, Parrino J, Campbell BV, Su SC, Pagnoni MF,
Stek JE, Xu J, Annunziato PW, Chan IS, Silber JL. Concomitant administration of zoster
and pneumococcal vaccines in adults ≥60 years old. Hum Vaccines 2010; 6(11): 894-902.
139. Chlibek R, Pauksens K, Rombo L, van Rijckevorsel G, Richardus JH, Plassmann
G, Schwarz TF, Catteau G, Lal H, Heineman TC. Long-term immunogenicity and safety of
an investigational herpes zoster subunit vaccine in older adults. Vaccine 2016; 34: 863-8.
140. de Boer PT, Wilschut JC, Postma MJ. Cost-effectiveness of vaccination against herpes
zoster. Hum Vaccin Immunother 2014; 10(7): 2048-61.
141. Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of
vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine
2014; 32(15): 1645-53.
142. Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 Years.
Ann Intern Med 2015; 163(7): 489-97.
143. Advisory Committee on Immunization Practices (ACIP) Summary Report February 24-25,
2010. https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-feb10.pdf
144. Immunize Canada. Herpes Zoster (Shingles). Available:
http://www.immunize.ca/en/diseases-vaccines/herpeszoster.aspx
145. Ontario. Ontario Making Shingles Vaccine Free for Seniors. Available:
https://news.ontario.ca/mohltc/en/2016/09/ontario-making-shingles-vaccine-free-for-seniors.html
49