よむ、つかう、まなぶ。
09参考資料1-3 9価ヒトパピローマウイルス( HPV )ワクチン ファクトシート (43 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000192554_00024.html |
| 出典情報 | 厚生科学審議会予防接種・ワクチン分科会 予防接種基本方針部会(第49回 10/4)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
1696
CONTINENTS and REGIONS. HPV and Cervical Cancer in the World: 2007 Report.
1732
cancer and precancer: Implications for screening and vaccination in Japan. Cancer Sci 111(7):
1697
WHO/ICO information Centre on HPV and cervical cancer (HPV Information Centre) 25:
1733
2546-2557.
1698
C1-C26.
1734
67.
1699
56. X. Castellsague, S. d. S., T. Aguado, K.S. Louie, L. Bruni, J. Munoz, M. Diaz, K. Irwin,
1735
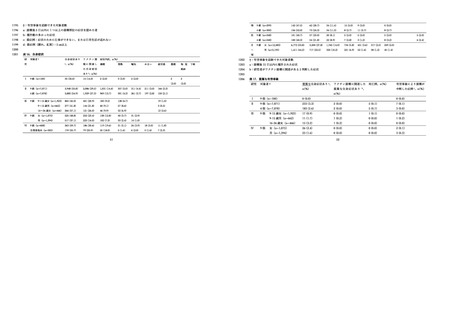
HPV16 に対する ELISA 抗体保有状況(2015-2019 年度).
1700
M. Gacic, O. Beauvais, G. Albero, E. Ferrer, S. Byrne, F.X. Bosch (2007). SECTION II.
1736
https://www.niid.go.jp/niid/ja/y-graphs/667-yosoku-graph.html
1701
COUNTRIES. HPV and Cervical Cancer in the World: 2007 Report. WHO/ICO information
1737
68. Arbyn, M., et al. (2018). Prophylactic vaccination against human papillomaviruses to
1702
Centre on HPV and cervical cancer (HPV Information Centre) 25: C27-C219.
1738
prevent cervical cancer and its precursors. Cochrane Database Syst Rev 5(5): Cd009069.
1703
57. X. Castellsague, S. d. S., T. Aguado, K.S. Louie, L. Bruni, J. Munoz, M. Diaz, K. Irwin,
1739
69. Schwarz, T. F., et al. (2019). A ten-year study of immunogenicity and safety of the AS04-
1704
M. Gacic, O. Beauvais, G. Albero, E. Ferrer, S. Byrne, F.X. Bosch (2007). SECTION III.
1740
HPV-16/18 vaccine in adolescent girls aged 10-14 years. Hum Vaccin Immunother 15(7-8):
1705
METHODS. HPV and Cervical Cancer in the World: 2007 Report. WHO/ICO information
1741
1970-1979.
1706
Centre on HPV and cervical cancer (HPV Information Centre) 25: C221-C230.
1742
70. Schwarz, T. F., et al. (2017). Ten-year immune persistence and safety of the HPV-16/18
1707
58.
de Sanjose, S., et al. (2010). Human papillomavirus genotype attribution in invasive
1743
AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer Med 6(11):
1708
cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11(11): 1048-
1744
2723-2731.
1709
1056. https://www.ncbi.nlm.nih.gov/pubmed/20952254
1745
71.
1710
59. Asato, T., et al. (2004). A large case-control study of cervical cancer risk associated with
1746
Preadolescents and Adolescents After 10 Years. Pediatrics 140(6).
1711
human papillomavirus infection in Japan, by nucleotide sequencing-based genotyping. J
1747
72.
1712
Infect Dis 189(10): 1829-1832. https://www.ncbi.nlm.nih.gov/pubmed/15122519
1748
efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of
1713
60. Onuki, M., et al. (2009). Human papillomavirus infections among Japanese women: age-
1749
three cohorts from randomized trials. BMJ Open 7(8): e015867.
1714
related prevalence and type-specific risk for cervical cancer. Cancer Sci 100(7): 1312-1316.
1750
73.
1715
https://www.ncbi.nlm.nih.gov/pubmed/19432906
1751
adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young
1716
61. Sasagawa, T., et al. (2016). Population-based study for human papillomavirus (HPV)
1752
Japanese women. Hum Vaccin Immunother 10(7): 1781-1794.
1717
infection in young women in Japan: A multicenter study by the Japanese human
1753
74. Yoshikawa, H., et al. (2013). Efficacy of quadrivalent human papillomavirus (types 6, 11,
1718
papillomavirus disease education research survey group (J-HERS). J Med Virol 88(2): 324-
1754
16 and 18) vaccine (GARDASIL) in Japanese women aged 18-26 years. Cancer Sci 104(4):
1719
335. https://www.ncbi.nlm.nih.gov/pubmed/26147986
1755
465-472.
1720
62.
Azuma, Y., et al. (2014). Human papillomavirus genotype distribution in cervical
1756
75.
1721
intraepithelial neoplasia grade 2/3 and invasive cervical cancer in Japanese women. Jpn J Clin
1757
long-term safety of the quadrivalent human papillomavirus vaccine in Japanese women. J
1722
Oncol 44(10): 910-917. https://www.ncbi.nlm.nih.gov/pubmed/25156680
1758
Infect Chemother 25(7): 520-525.
1723
63. Sakamoto, J., et al. (2018). Single type infection of human papillomavirus as a cause for
1759
76. Wheeler, C. M., et al. (2012). Cross-protective efficacy of HPV-16/18 AS04-adjuvanted
1724
high-grade cervical intraepithelial neoplasia and invasive cancer in Japan. Papillomavirus Res
1760
vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types:
1725
6: 46-51. https://www.ncbi.nlm.nih.gov/pubmed/30401640
1761
4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol
1726
64. Kondo, K., et al. (2012). Genotype distribution of human papillomaviruses in Japanese
1762
13(1): 100-110.
1727
women with abnormal cervical cytology. Open Virol J 6: 277-283.
1763
77. Tota, J. E., et al. (2020). Efficacy of the AS04-Adjuvanted HPV16/18 Vaccine: Pooled
1728
65. Torii, Y., et al. (2016). Comparison of methods using paraffin-embedded tissues and
1764
Analysis of the Costa Rica Vaccine and PATRICIA Randomized Controlled Trials. J Natl
1729
exfoliated cervical cells to evaluate human papillomavirus genotype attribution. Cancer Sci
1765
Cancer Inst 112(8): 818-828.
1730
107(10): 1520-1526. https://www.ncbi.nlm.nih.gov/pubmed/27501394
1766
78.
1731
66.
1767
vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12(10): 781-789.
Onuki, M., et al. (2020). Human papillomavirus genotype contribution to cervical
85
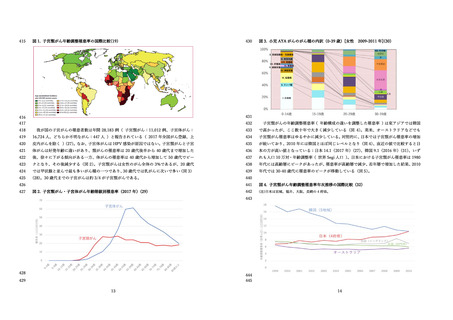
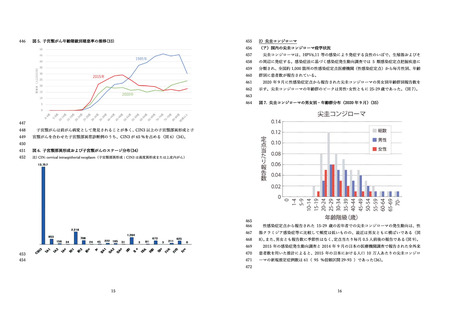
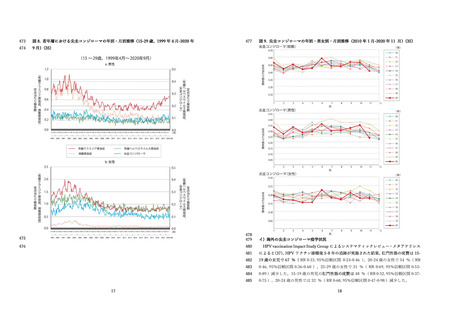
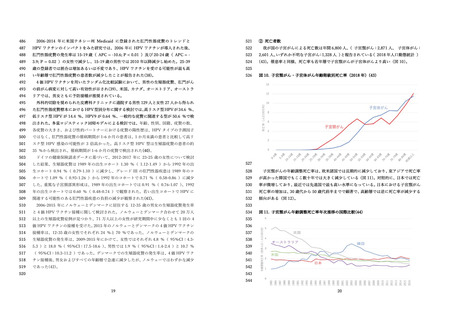
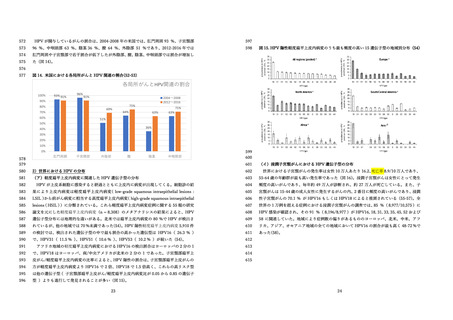
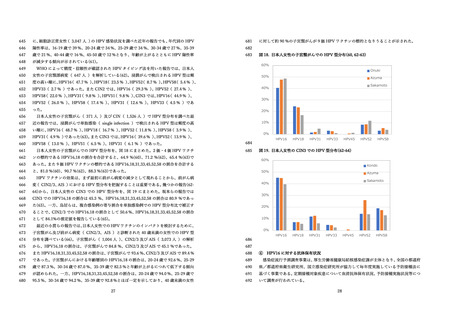
国⽴感染症研究所 感染症流⾏予測調査.(2020).感染症流⾏予測調査事業に基づく
Ferris, D. G., et al. (2017). 4-Valent Human Papillomavirus (4vHPV) Vaccine in
Lehtinen, M., et al. (2017). Ten-year follow-up of human papillomavirus vaccine
Konno, R., et al. (2014). Efficacy of the human papillomavirus (HPV)-16/18 AS04-
Sakamoto, M., et al. (2019). Effectiveness on high-grade cervical abnormalities and
Malagón, T., et al. (2012). Cross-protective efficacy of two human papillomavirus
86
CONTINENTS and REGIONS. HPV and Cervical Cancer in the World: 2007 Report.
1732
cancer and precancer: Implications for screening and vaccination in Japan. Cancer Sci 111(7):
1697
WHO/ICO information Centre on HPV and cervical cancer (HPV Information Centre) 25:
1733
2546-2557.
1698
C1-C26.
1734
67.
1699
56. X. Castellsague, S. d. S., T. Aguado, K.S. Louie, L. Bruni, J. Munoz, M. Diaz, K. Irwin,
1735
HPV16 に対する ELISA 抗体保有状況(2015-2019 年度).
1700
M. Gacic, O. Beauvais, G. Albero, E. Ferrer, S. Byrne, F.X. Bosch (2007). SECTION II.
1736
https://www.niid.go.jp/niid/ja/y-graphs/667-yosoku-graph.html
1701
COUNTRIES. HPV and Cervical Cancer in the World: 2007 Report. WHO/ICO information
1737
68. Arbyn, M., et al. (2018). Prophylactic vaccination against human papillomaviruses to
1702
Centre on HPV and cervical cancer (HPV Information Centre) 25: C27-C219.
1738
prevent cervical cancer and its precursors. Cochrane Database Syst Rev 5(5): Cd009069.
1703
57. X. Castellsague, S. d. S., T. Aguado, K.S. Louie, L. Bruni, J. Munoz, M. Diaz, K. Irwin,
1739
69. Schwarz, T. F., et al. (2019). A ten-year study of immunogenicity and safety of the AS04-
1704
M. Gacic, O. Beauvais, G. Albero, E. Ferrer, S. Byrne, F.X. Bosch (2007). SECTION III.
1740
HPV-16/18 vaccine in adolescent girls aged 10-14 years. Hum Vaccin Immunother 15(7-8):
1705
METHODS. HPV and Cervical Cancer in the World: 2007 Report. WHO/ICO information
1741
1970-1979.
1706
Centre on HPV and cervical cancer (HPV Information Centre) 25: C221-C230.
1742
70. Schwarz, T. F., et al. (2017). Ten-year immune persistence and safety of the HPV-16/18
1707
58.
de Sanjose, S., et al. (2010). Human papillomavirus genotype attribution in invasive
1743
AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer Med 6(11):
1708
cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11(11): 1048-
1744
2723-2731.
1709
1056. https://www.ncbi.nlm.nih.gov/pubmed/20952254
1745
71.
1710
59. Asato, T., et al. (2004). A large case-control study of cervical cancer risk associated with
1746
Preadolescents and Adolescents After 10 Years. Pediatrics 140(6).
1711
human papillomavirus infection in Japan, by nucleotide sequencing-based genotyping. J
1747
72.
1712
Infect Dis 189(10): 1829-1832. https://www.ncbi.nlm.nih.gov/pubmed/15122519
1748
efficacy against the most stringent cervical neoplasia end-point-registry-based follow-up of
1713
60. Onuki, M., et al. (2009). Human papillomavirus infections among Japanese women: age-
1749
three cohorts from randomized trials. BMJ Open 7(8): e015867.
1714
related prevalence and type-specific risk for cervical cancer. Cancer Sci 100(7): 1312-1316.
1750
73.
1715
https://www.ncbi.nlm.nih.gov/pubmed/19432906
1751
adjuvanted vaccine against cervical intraepithelial neoplasia and cervical infection in young
1716
61. Sasagawa, T., et al. (2016). Population-based study for human papillomavirus (HPV)
1752
Japanese women. Hum Vaccin Immunother 10(7): 1781-1794.
1717
infection in young women in Japan: A multicenter study by the Japanese human
1753
74. Yoshikawa, H., et al. (2013). Efficacy of quadrivalent human papillomavirus (types 6, 11,
1718
papillomavirus disease education research survey group (J-HERS). J Med Virol 88(2): 324-
1754
16 and 18) vaccine (GARDASIL) in Japanese women aged 18-26 years. Cancer Sci 104(4):
1719
335. https://www.ncbi.nlm.nih.gov/pubmed/26147986
1755
465-472.
1720
62.
Azuma, Y., et al. (2014). Human papillomavirus genotype distribution in cervical
1756
75.
1721
intraepithelial neoplasia grade 2/3 and invasive cervical cancer in Japanese women. Jpn J Clin
1757
long-term safety of the quadrivalent human papillomavirus vaccine in Japanese women. J
1722
Oncol 44(10): 910-917. https://www.ncbi.nlm.nih.gov/pubmed/25156680
1758
Infect Chemother 25(7): 520-525.
1723
63. Sakamoto, J., et al. (2018). Single type infection of human papillomavirus as a cause for
1759
76. Wheeler, C. M., et al. (2012). Cross-protective efficacy of HPV-16/18 AS04-adjuvanted
1724
high-grade cervical intraepithelial neoplasia and invasive cancer in Japan. Papillomavirus Res
1760
vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types:
1725
6: 46-51. https://www.ncbi.nlm.nih.gov/pubmed/30401640
1761
4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol
1726
64. Kondo, K., et al. (2012). Genotype distribution of human papillomaviruses in Japanese
1762
13(1): 100-110.
1727
women with abnormal cervical cytology. Open Virol J 6: 277-283.
1763
77. Tota, J. E., et al. (2020). Efficacy of the AS04-Adjuvanted HPV16/18 Vaccine: Pooled
1728
65. Torii, Y., et al. (2016). Comparison of methods using paraffin-embedded tissues and
1764
Analysis of the Costa Rica Vaccine and PATRICIA Randomized Controlled Trials. J Natl
1729
exfoliated cervical cells to evaluate human papillomavirus genotype attribution. Cancer Sci
1765
Cancer Inst 112(8): 818-828.
1730
107(10): 1520-1526. https://www.ncbi.nlm.nih.gov/pubmed/27501394
1766
78.
1731
66.
1767
vaccines: a systematic review and meta-analysis. Lancet Infect Dis 12(10): 781-789.
Onuki, M., et al. (2020). Human papillomavirus genotype contribution to cervical
85
国⽴感染症研究所 感染症流⾏予測調査.(2020).感染症流⾏予測調査事業に基づく
Ferris, D. G., et al. (2017). 4-Valent Human Papillomavirus (4vHPV) Vaccine in
Lehtinen, M., et al. (2017). Ten-year follow-up of human papillomavirus vaccine
Konno, R., et al. (2014). Efficacy of the human papillomavirus (HPV)-16/18 AS04-
Sakamoto, M., et al. (2019). Effectiveness on high-grade cervical abnormalities and
Malagón, T., et al. (2012). Cross-protective efficacy of two human papillomavirus
86