よむ、つかう、まなぶ。
資料1-3 ワクチンの安全性に関する評価について (18 ページ)
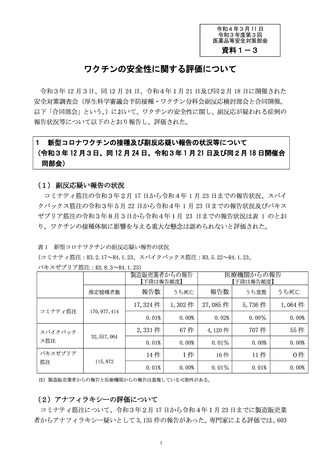
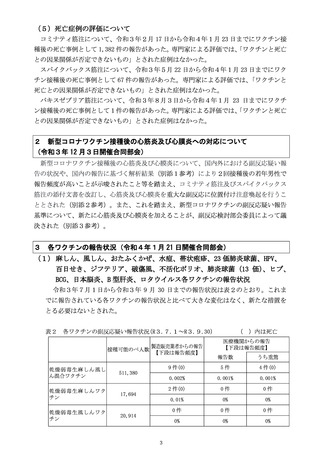
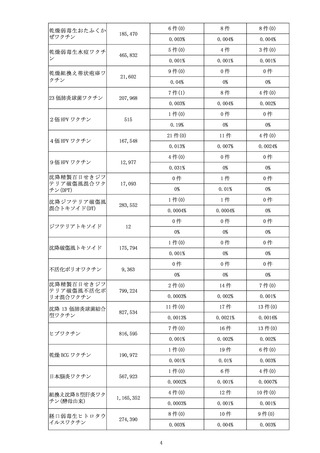
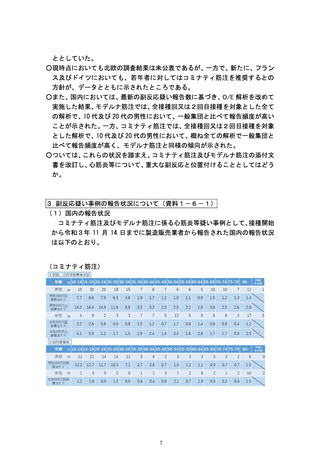
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_24331.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会(令和3年度 第3回 3/11)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
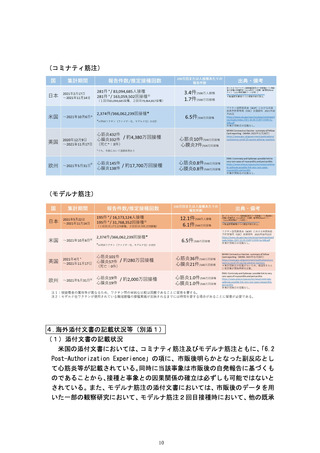
authorized or approved mRNA COVID-19
pain, shortness of breath, or palpitations
vaccines. Although postmarketing data
following vaccination.
following a booster dose of mRNA vaccines
Healthcare professionals should consult
are limited, available evidence suggests a
guidance and/or specialists to diagnose and
lower myocarditis risk following a booster
treat this condition.
dose relative to the risk following the primary
The risk of myocarditis after a third dose (0.5
series second dose.
mL, 100 micrograms) or booster dose (0.25
The CDC has published considerations related
mL, 50 micrograms) of Spikevax has not yet
to myocarditis and pericarditis after
been characterised.
vaccination, including for vaccination of
individuals with a history of myocarditis or
pericarditis
(https://www.cdc.gov/vaccines/covid19/clinical-considerations/myocarditis.html).
6 OVERALL SAFETY SUMMARY
4.8 Undesirable effects
4.8 Undesirable effects
Anaphylaxis and other severe allergic
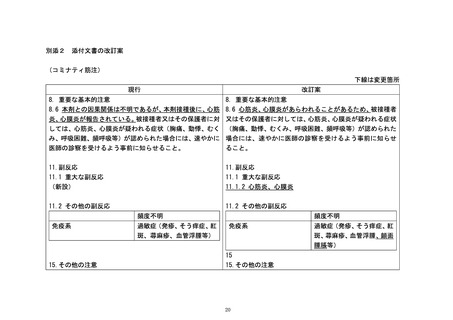
Table 1 Adverse reactions from Spikevax
Table 1: Adverse reactions from Spikevax
reactions, myocarditis, pericarditis, and
clinical trials and post-authorisation
clinical trials and post authorisation
syncope have been reported following
experience in individuals 12 years of age
experience in individuals 12 years of age
administration of the Moderna COVID-19
and older
and older
Vaccine outside of clinical trials..
Cardiac disorders
Cardiac disorders
18
pain, shortness of breath, or palpitations
vaccines. Although postmarketing data
following vaccination.
following a booster dose of mRNA vaccines
Healthcare professionals should consult
are limited, available evidence suggests a
guidance and/or specialists to diagnose and
lower myocarditis risk following a booster
treat this condition.
dose relative to the risk following the primary
The risk of myocarditis after a third dose (0.5
series second dose.
mL, 100 micrograms) or booster dose (0.25
The CDC has published considerations related
mL, 50 micrograms) of Spikevax has not yet
to myocarditis and pericarditis after
been characterised.
vaccination, including for vaccination of
individuals with a history of myocarditis or
pericarditis
(https://www.cdc.gov/vaccines/covid19/clinical-considerations/myocarditis.html).
6 OVERALL SAFETY SUMMARY
4.8 Undesirable effects
4.8 Undesirable effects
Anaphylaxis and other severe allergic
Table 1 Adverse reactions from Spikevax
Table 1: Adverse reactions from Spikevax
reactions, myocarditis, pericarditis, and
clinical trials and post-authorisation
clinical trials and post authorisation
syncope have been reported following
experience in individuals 12 years of age
experience in individuals 12 years of age
administration of the Moderna COVID-19
and older
and older
Vaccine outside of clinical trials..
Cardiac disorders
Cardiac disorders
18