よむ、つかう、まなぶ。
資料2-2 調査結果報告書 (26 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_24579.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和3年度第31回 3/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
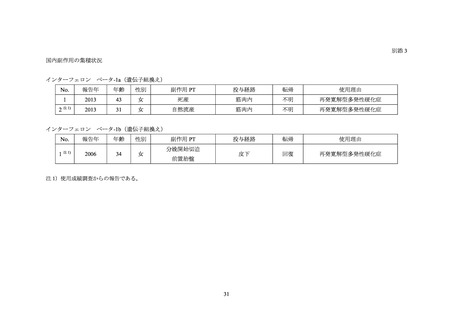
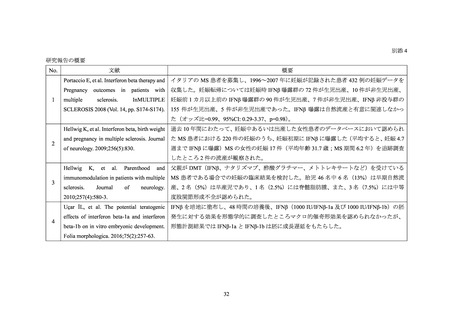
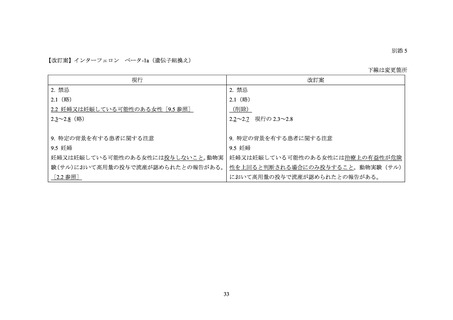
別添 2
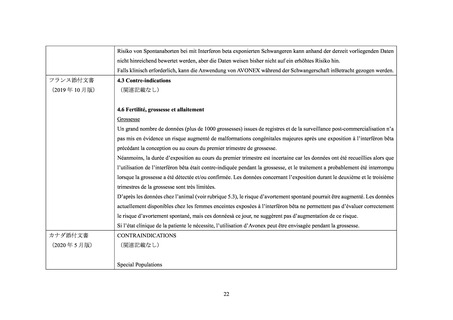
海外添付文書の記載状況(ベタフェロン)
米国添付文書
4. CONTRAINDICATIONS
(2020 年 10 月版)
(関連記載なし)
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Although there have been no well-controlled studies in pregnant women, available data, which includes prospective
observational studies, have not generally indicated a drug-associated risk of major birth defects with interferon beta-1b
during pregnancy. Administration of BETASERON to monkeys during gestation resulted in increased embryo-fetal death
at or above exposures greater than 3 times the human therapeutic dose (see Animal Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized
pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated
population is unknown.
Data
Human Data
The majority of the observational studies reporting on pregnancies exposed to interferon beta-1b did not identify an
association between the use of interferon beta-1b during pregnancy and an increased risk of major birth defects.
Animal Data
When BETASERON (doses ranging from 0.028 to 0.42 mg/kg/day) was administered to pregnant rhesus monkeys
throughout the period of organogenesis (gestation days 20 to 70), a dose-related abortifacient effect was observed. The low-
26
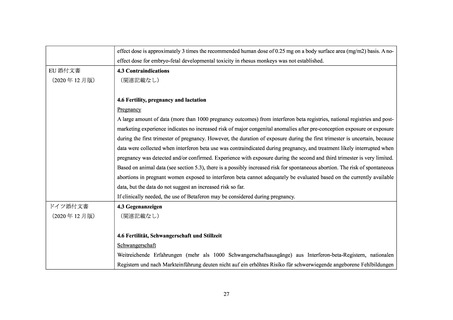
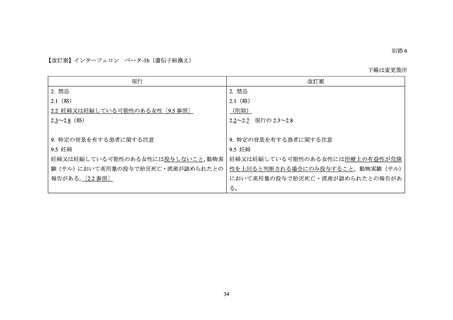
海外添付文書の記載状況(ベタフェロン)
米国添付文書
4. CONTRAINDICATIONS
(2020 年 10 月版)
(関連記載なし)
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Although there have been no well-controlled studies in pregnant women, available data, which includes prospective
observational studies, have not generally indicated a drug-associated risk of major birth defects with interferon beta-1b
during pregnancy. Administration of BETASERON to monkeys during gestation resulted in increased embryo-fetal death
at or above exposures greater than 3 times the human therapeutic dose (see Animal Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized
pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated
population is unknown.
Data
Human Data
The majority of the observational studies reporting on pregnancies exposed to interferon beta-1b did not identify an
association between the use of interferon beta-1b during pregnancy and an increased risk of major birth defects.
Animal Data
When BETASERON (doses ranging from 0.028 to 0.42 mg/kg/day) was administered to pregnant rhesus monkeys
throughout the period of organogenesis (gestation days 20 to 70), a dose-related abortifacient effect was observed. The low-
26