よむ、つかう、まなぶ。
資料2-2 調査結果報告書 (20 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_24579.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和3年度第31回 3/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
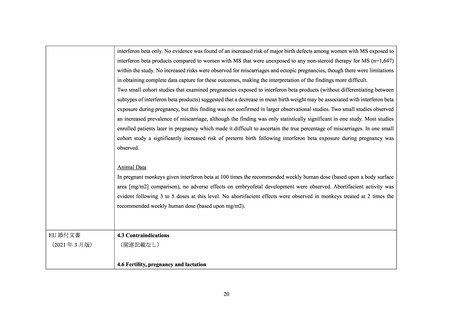
interferon beta only. No evidence was found of an increased risk of major birth defects among women with MS exposed to
interferon beta products compared to women with MS that were unexposed to any non-steroid therapy for MS (n=1,647)
within the study. No increased risks were observed for miscarriages and ectopic pregnancies, though there were limitations
in obtaining complete data capture for these outcomes, making the interpretation of the findings more difficult.
Two small cohort studies that examined pregnancies exposed to interferon beta products (without differentiating between
subtypes of interferon beta products) suggested that a decrease in mean birth weight may be associated with interferon beta
exposure during pregnancy, but this finding was not confirmed in larger observational studies. Two small studies observed
an increased prevalence of miscarriage, although the finding was only statistically significant in one study. Most studies
enrolled patients later in pregnancy which made it difficult to ascertain the true percentage of miscarriages. In one small
cohort study a significantly increased risk of preterm birth following interferon beta exposure during pregnancy was
observed.
Animal Data
In pregnant monkeys given interferon beta at 100 times the recommended weekly human dose (based upon a body surface
area [mg/m2] comparison), no adverse effects on embryofetal development were observed. Abortifacient activity was
evident following 3 to 5 doses at this level. No abortifacient effects were observed in monkeys treated at 2 times the
recommended weekly human dose (based upon mg/m2).
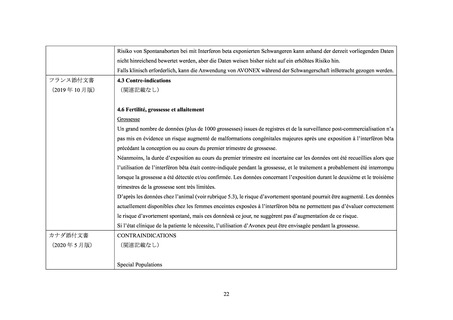
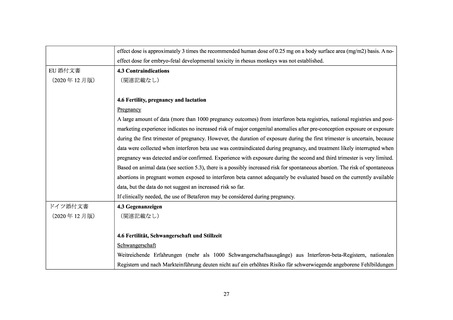
EU 添付文書
4.3 Contraindications
(2021 年 3 月版)
(関連記載なし)
4.6 Fertility, pregnancy and lactation
20
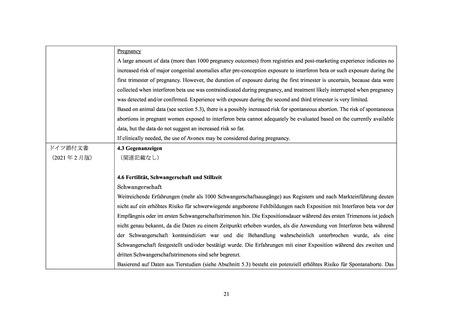
interferon beta products compared to women with MS that were unexposed to any non-steroid therapy for MS (n=1,647)
within the study. No increased risks were observed for miscarriages and ectopic pregnancies, though there were limitations
in obtaining complete data capture for these outcomes, making the interpretation of the findings more difficult.
Two small cohort studies that examined pregnancies exposed to interferon beta products (without differentiating between
subtypes of interferon beta products) suggested that a decrease in mean birth weight may be associated with interferon beta
exposure during pregnancy, but this finding was not confirmed in larger observational studies. Two small studies observed
an increased prevalence of miscarriage, although the finding was only statistically significant in one study. Most studies
enrolled patients later in pregnancy which made it difficult to ascertain the true percentage of miscarriages. In one small
cohort study a significantly increased risk of preterm birth following interferon beta exposure during pregnancy was
observed.
Animal Data
In pregnant monkeys given interferon beta at 100 times the recommended weekly human dose (based upon a body surface
area [mg/m2] comparison), no adverse effects on embryofetal development were observed. Abortifacient activity was
evident following 3 to 5 doses at this level. No abortifacient effects were observed in monkeys treated at 2 times the
recommended weekly human dose (based upon mg/m2).
EU 添付文書
4.3 Contraindications
(2021 年 3 月版)
(関連記載なし)
4.6 Fertility, pregnancy and lactation
20