よむ、つかう、まなぶ。
資料2-2 調査結果報告書 (23 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_24579.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和3年度第31回 3/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
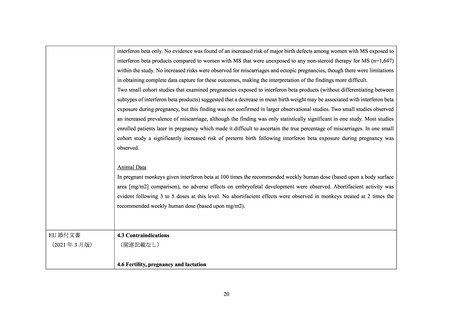
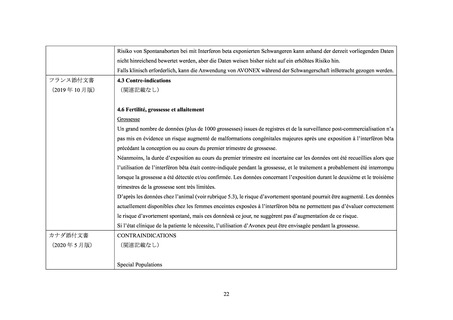
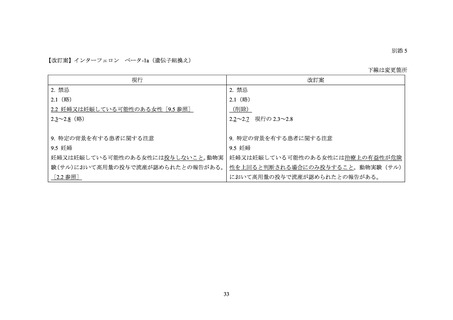
Pregnant Women:
The extent of exposure in pregnancy during clinical trials is:limited: < 1000 pregnancies
There are no adequate and well-controlled studies of AVONEX PS/AVONEX PEN in pregnant women. The administration
of AVONEX PS/AVONEX PEN during confirmed pregnancy should be avoided, unless clearly needed.
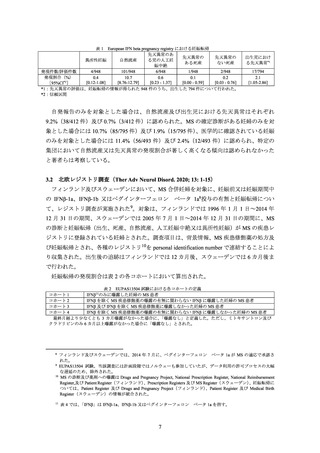
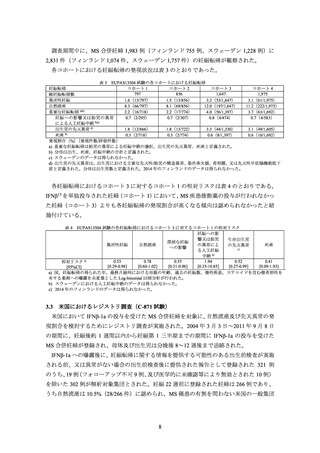
A European registry study collected data on 948 prospective pregnancies in women with MS who were treated with one of
five interferon beta medications. The rates of aggregated adverse pregnancy outcomes were in line with reference ranges
published in the literature.
Data from a prospective pregnancy registry that included 302 MS patients exposed to Avonex in the United States found an
increased incidence of major birth defects compared to a reference population. Data from a retrospective register-based
study in Sweden and Finland have not indicated an increased risk of major congenital anomalies after early pregnancy
exposure to drugs in the interferon beta class. Given these contrasting data, it is unclear whether Avonex has teratogenic
effects.
In each of the studies discussed above, the duration of exposure during the first trimester was uncertain since data were
collected when interferon beta use was contraindicated or strongly advised against during pregnancy, and treatment was
interrupted when the pregnancy was detected and/or confirmed. Experience with exposure during the second and third
trimester was too limited to determine whether exposure affects maternal or fetal health.
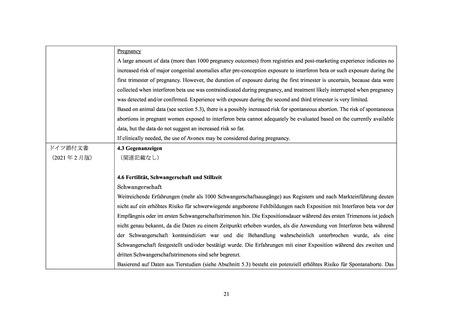
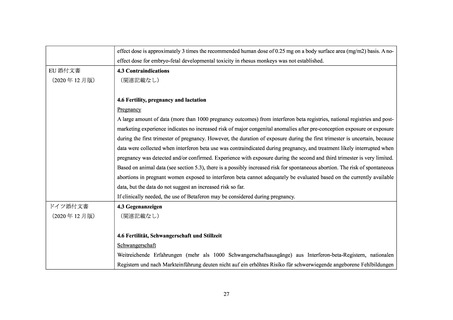
The reproductive toxicity of AVONEX PS/AVONEX PEN has been studied in animals. In pregnant monkeys given
AVONEX at 100 times the recommended weekly human dose (based upon body surface area comparison), no teratogenic
23
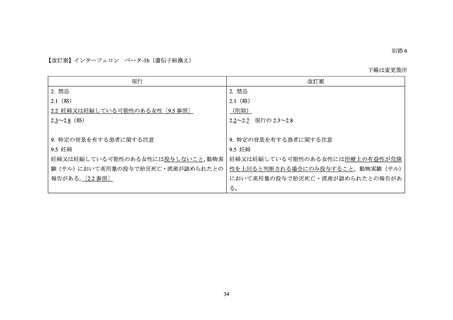
The extent of exposure in pregnancy during clinical trials is:limited: < 1000 pregnancies
There are no adequate and well-controlled studies of AVONEX PS/AVONEX PEN in pregnant women. The administration
of AVONEX PS/AVONEX PEN during confirmed pregnancy should be avoided, unless clearly needed.
A European registry study collected data on 948 prospective pregnancies in women with MS who were treated with one of
five interferon beta medications. The rates of aggregated adverse pregnancy outcomes were in line with reference ranges
published in the literature.
Data from a prospective pregnancy registry that included 302 MS patients exposed to Avonex in the United States found an
increased incidence of major birth defects compared to a reference population. Data from a retrospective register-based
study in Sweden and Finland have not indicated an increased risk of major congenital anomalies after early pregnancy
exposure to drugs in the interferon beta class. Given these contrasting data, it is unclear whether Avonex has teratogenic
effects.
In each of the studies discussed above, the duration of exposure during the first trimester was uncertain since data were
collected when interferon beta use was contraindicated or strongly advised against during pregnancy, and treatment was
interrupted when the pregnancy was detected and/or confirmed. Experience with exposure during the second and third
trimester was too limited to determine whether exposure affects maternal or fetal health.
The reproductive toxicity of AVONEX PS/AVONEX PEN has been studied in animals. In pregnant monkeys given
AVONEX at 100 times the recommended weekly human dose (based upon body surface area comparison), no teratogenic
23