よむ、つかう、まなぶ。
参考資料2 新型コロナワクチンの接種について(令和4年10月7日第38回厚生科学審議会予防接種・ワクチン分科会資料1) (54 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000208910_00053.html |
| 出典情報 | 第87回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第16回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
参考資料一覧(1/5)
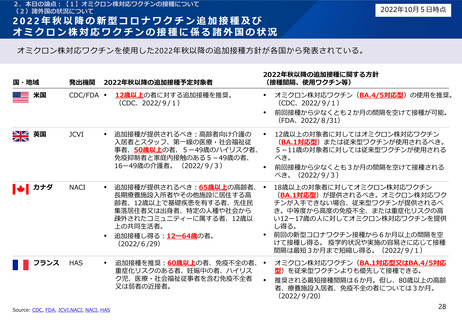
2022年秋以降の新型コロナワクチン追加接種及びオミクロン株対応ワクチンの接種に係る諸外国の状況
米国
CDC. 2022. CDC Recommends the First Updated COVID-19 Booster. [online] Available at: <https://www.cdc.gov/media/releases/2022/s0901-covid-19-booster.html> [Accessed 13 Sep 2022].
U.S. Food and Drug Administration. 2022. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. [online] Available at: <https://www.fda.gov/news-events/pressannouncements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use> [Accessed 13 Sep 2022].
英国
GOV.UK. 2022. JCVI statement on the COVID-19 booster vaccination programme for autumn 2022: update 3 September 2022. [online] Available at: <https://www.gov.uk/government/publications/covid-19-vaccines-for-autumn2022-jcvi-advice-15-august-2022/jcvi-statement-on-the-covid-19-booster-vaccination-programme-for-autumn-2022-update-15-august-2022> [Accessed Sep 13, 2022].
カナダ
NACI. 2022. Recommendations on the use of bivalent Omicroncontaining mRNA COVID-19 vaccines. [online] Available at:
<https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-bivalent-Omicron-containing-mrna-covid-19-vaccines.pdf> [Accessed 13 Sep 2022].
NACI. 2022. Interim guidance on planning considerations for a fall 2022 COVID-19 vaccine booster program in Canada. [online] Available at: <https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisorycommittee-on-immunization-naci/naci-guidance-planning-fall-2022-covid-19-vaccine-booster.pdf> [Accessed 13 Sep 2022].
フランス
Haute Autorité de Santé. 2022. Stratégie vaccinale de rappel contre la Covid-19. [online] Available at: <https://www.has-sante.fr/jcms/p_3367885/fr/strategie-vaccinale-de-rappel-contre-la-covid-19> [Accessed 5 Oct 2022].
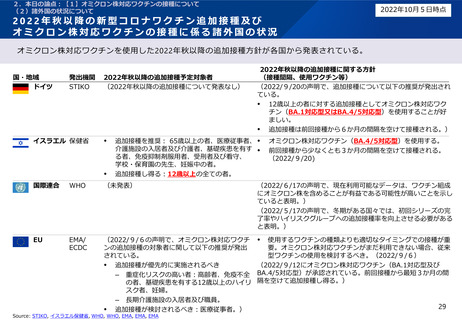
ドイツ
STIKO. 2022. Pressemitteilung der STIKO zur 22. Aktualisierung der COVID-19-Impfempfehlung. https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/PM_2022-09-20.html [Accessed Oct
5, 2022]
イスラエル
Ministry of Health. 2022. HMOs Roll Out an Omicron-Specific Vaccine. [online] Available at: < https://www.gov.il/en/departments/news/20092022-04 > [Accessed 5 Oct 2022].
国際連合
WHO. 2022. Interim statement on the composition of current COVID-19 vaccines. [online] Available at: <https://www.who.int/news/item/17-06-2022-interim-statement-on--the-composition-of-current-COVID-19-vaccines> [Accessed 21 July 2022].
WHO. 2022. Interim statement on the use of additional booster doses of Emergency Use Listed mRNA vaccines against COVID-19. [online] Available at: <https://www.who.int/news/item/17-05-2022-interim-statement-on-the-use-of-additionalbooster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19> [Accessed 21 July 2022].
EU
EMA. 2022. ECDC-EMA statement on booster vaccination with Omicron adapted bivalent COVID-19 vaccines. [online] Available at: < https://www.ema.europa.eu/en/documents/public-statement/ecdc-ema-statement-booster-vaccination-omicronadapted-bivalent-covid-19-vaccines_-0.pdf > [Accessed 13 Sep 2022].
EMA. 2022. Comirnaty. [online] Available at: <https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty > [Accessed 5 Oct 2022].
EMA. 2022. Spikevax (previously COVID-19 Vaccine Moderna). [online] Available at: <https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax> [Accessed 5 Oct 2022].
54
2022年秋以降の新型コロナワクチン追加接種及びオミクロン株対応ワクチンの接種に係る諸外国の状況
米国
CDC. 2022. CDC Recommends the First Updated COVID-19 Booster. [online] Available at: <https://www.cdc.gov/media/releases/2022/s0901-covid-19-booster.html> [Accessed 13 Sep 2022].
U.S. Food and Drug Administration. 2022. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. [online] Available at: <https://www.fda.gov/news-events/pressannouncements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use> [Accessed 13 Sep 2022].
英国
GOV.UK. 2022. JCVI statement on the COVID-19 booster vaccination programme for autumn 2022: update 3 September 2022. [online] Available at: <https://www.gov.uk/government/publications/covid-19-vaccines-for-autumn2022-jcvi-advice-15-august-2022/jcvi-statement-on-the-covid-19-booster-vaccination-programme-for-autumn-2022-update-15-august-2022> [Accessed Sep 13, 2022].
カナダ
NACI. 2022. Recommendations on the use of bivalent Omicroncontaining mRNA COVID-19 vaccines. [online] Available at:
<https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-bivalent-Omicron-containing-mrna-covid-19-vaccines.pdf> [Accessed 13 Sep 2022].
NACI. 2022. Interim guidance on planning considerations for a fall 2022 COVID-19 vaccine booster program in Canada. [online] Available at: <https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisorycommittee-on-immunization-naci/naci-guidance-planning-fall-2022-covid-19-vaccine-booster.pdf> [Accessed 13 Sep 2022].
フランス
Haute Autorité de Santé. 2022. Stratégie vaccinale de rappel contre la Covid-19. [online] Available at: <https://www.has-sante.fr/jcms/p_3367885/fr/strategie-vaccinale-de-rappel-contre-la-covid-19> [Accessed 5 Oct 2022].
ドイツ
STIKO. 2022. Pressemitteilung der STIKO zur 22. Aktualisierung der COVID-19-Impfempfehlung. https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/PM_2022-09-20.html [Accessed Oct
5, 2022]
イスラエル
Ministry of Health. 2022. HMOs Roll Out an Omicron-Specific Vaccine. [online] Available at: < https://www.gov.il/en/departments/news/20092022-04 > [Accessed 5 Oct 2022].
国際連合
WHO. 2022. Interim statement on the composition of current COVID-19 vaccines. [online] Available at: <https://www.who.int/news/item/17-06-2022-interim-statement-on--the-composition-of-current-COVID-19-vaccines> [Accessed 21 July 2022].
WHO. 2022. Interim statement on the use of additional booster doses of Emergency Use Listed mRNA vaccines against COVID-19. [online] Available at: <https://www.who.int/news/item/17-05-2022-interim-statement-on-the-use-of-additionalbooster-doses-of-emergency-use-listed-mrna-vaccines-against-covid-19> [Accessed 21 July 2022].
EU
EMA. 2022. ECDC-EMA statement on booster vaccination with Omicron adapted bivalent COVID-19 vaccines. [online] Available at: < https://www.ema.europa.eu/en/documents/public-statement/ecdc-ema-statement-booster-vaccination-omicronadapted-bivalent-covid-19-vaccines_-0.pdf > [Accessed 13 Sep 2022].
EMA. 2022. Comirnaty. [online] Available at: <https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty > [Accessed 5 Oct 2022].
EMA. 2022. Spikevax (previously COVID-19 Vaccine Moderna). [online] Available at: <https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax> [Accessed 5 Oct 2022].
54