よむ、つかう、まなぶ。
参考資料1-2: 令和3年度厚生労働科学特別研究事業「臨床研究法の見直しの審議における新たな課題・論点への対応策の確立のための研究」班 欧米での観察研究の取り扱い (15 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_24643.html |
| 出典情報 | 厚生科学審議会 臨床研究部会(第29回 3/24)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
i
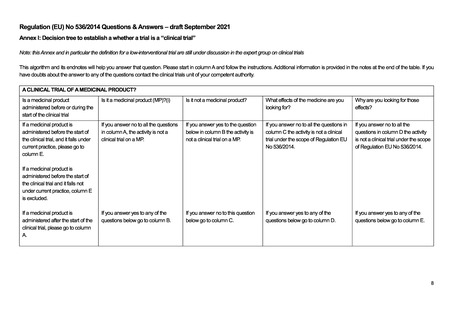
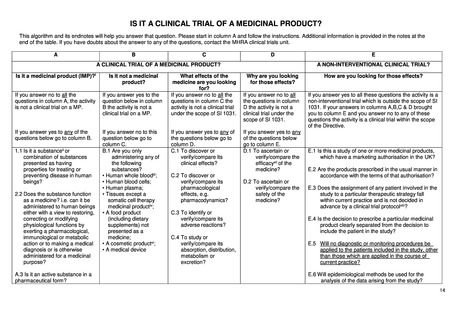
Article 2 of The Human Medicines Regulations 2012 provides the definition of "medicinal product."
Substance is any matter irrespective of origin e.g. human, animal, vegetable or chemical that is being administered to a human being.
iii This does not include derivatives of human whole blood, human blood cells and human plasma that involve a manufacturing process.
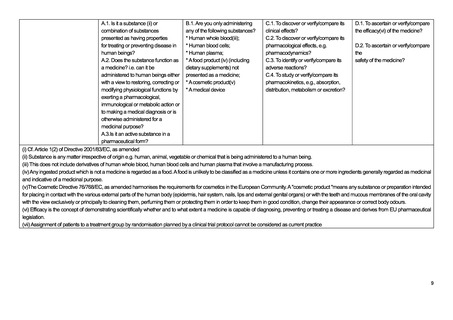
iv Somatic cell therapy medicinal products use somatic living cells of human (or animal) origin, the biological characteristics of which have been substantially altered as a
result of their manipulation to obtain a therapeutic, diagnostic or preventative effect (in humans) through metabolic, pharmacological and immunological means.
v Any ingested product which is not a medicine is regarded as a food. A food is unlikely to be classified as a medicine unless it contains one or more ingredients generally
regarded as medicinal and indicative of a medicinal purpose.
vi The Cosmetic Directive 76/768/EC, as amended harmonises the requirements for cosmetics in the European Community. A "cosmetic product "means any substance or
preparation intended for placing in contact with the various external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth
and mucous membranes of the oral cavity with the view exclusively or principally to cleaning them, perfuming them or protecting them in order to keep them in good condition,
change their appearance or correct body odours.
vii Efficacy is the concept of demonstrating scientifically whether and to what extent a medicine is capable of diagnosing, preventing or treating a disease.
viii Assignment of patients to a treatment group by randomisation planned by a clinical trial protocol cannot be considered as current practice.
ii
15
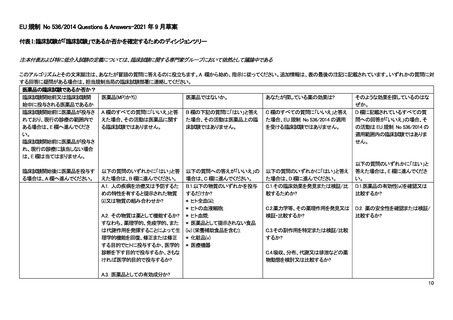
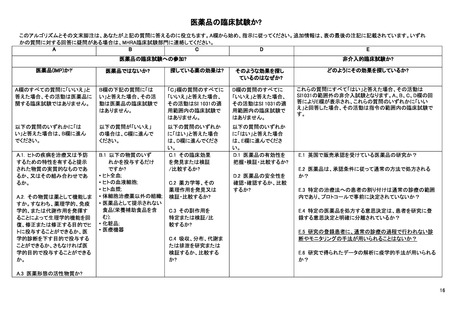
Article 2 of The Human Medicines Regulations 2012 provides the definition of "medicinal product."
Substance is any matter irrespective of origin e.g. human, animal, vegetable or chemical that is being administered to a human being.
iii This does not include derivatives of human whole blood, human blood cells and human plasma that involve a manufacturing process.
iv Somatic cell therapy medicinal products use somatic living cells of human (or animal) origin, the biological characteristics of which have been substantially altered as a
result of their manipulation to obtain a therapeutic, diagnostic or preventative effect (in humans) through metabolic, pharmacological and immunological means.
v Any ingested product which is not a medicine is regarded as a food. A food is unlikely to be classified as a medicine unless it contains one or more ingredients generally
regarded as medicinal and indicative of a medicinal purpose.
vi The Cosmetic Directive 76/768/EC, as amended harmonises the requirements for cosmetics in the European Community. A "cosmetic product "means any substance or
preparation intended for placing in contact with the various external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth
and mucous membranes of the oral cavity with the view exclusively or principally to cleaning them, perfuming them or protecting them in order to keep them in good condition,
change their appearance or correct body odours.
vii Efficacy is the concept of demonstrating scientifically whether and to what extent a medicine is capable of diagnosing, preventing or treating a disease.
viii Assignment of patients to a treatment group by randomisation planned by a clinical trial protocol cannot be considered as current practice.
ii
15