よむ、つかう、まなぶ。
参考資料1-2: 令和3年度厚生労働科学特別研究事業「臨床研究法の見直しの審議における新たな課題・論点への対応策の確立のための研究」班 欧米での観察研究の取り扱い (14 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_24643.html |
| 出典情報 | 厚生科学審議会 臨床研究部会(第29回 3/24)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
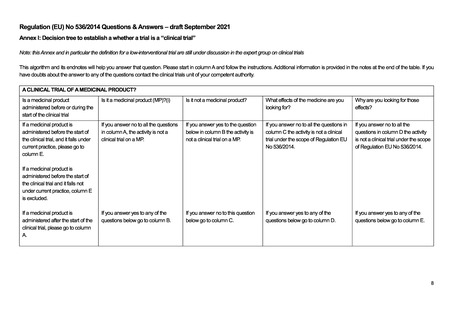
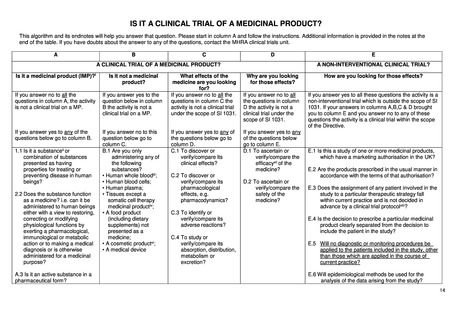
IS IT A CLINICAL TRIAL OF A MEDICINAL PRODUCT?
This algorithm and its endnotes will help you answer that question. Please start in column A and follow the instructions. Additional information is provided in the notes at the
end of the table. If you have doubts about the answer to any of the questions, contact the MHRA clinical trials unit.
A
B
C
D
A CLINICAL TRIAL OF A MEDICINAL PRODUCT?
Is it a medicinal product (IMP)?i
Is it not a medicinal
product?
If you answer no to all the
questions in column A, the activity
is not a clinical trial on a MP.
If you answer yes to the
question below in column
B the activity is not a
clinical trial on a MP.
If you answer yes to any of the
questions below go to column B.
If you answer no to this
question below go to
column C.
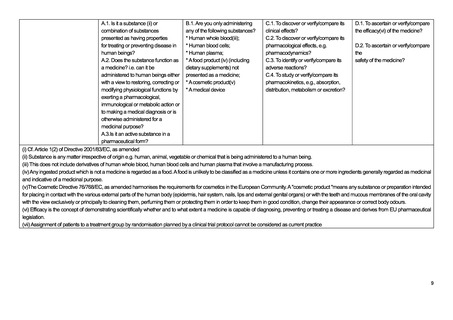
B.1 Are you only
administering any of
the following
substances?
• Human whole bloodiii;
• Human blood cells;
• Human plasma;
• Tissues except a
somatic cell therapy
medicinal productiv;
• A food product
(including dietary
supplements) not
presented as a
medicine;
• A cosmetic productvi;
• A medical device
1.1 Is it a substanceii or
combination of substances
presented as having
properties for treating or
preventing disease in human
beings?
2.2 Does the substance function
as a medicine? i.e. can it be
administered to human beings
either with a view to restoring,
correcting or modifying
physiological functions by
exerting a pharmacological,
immunological or metabolic
action or to making a medical
diagnosis or is otherwise
administered for a medicinal
purpose?
A.3 Is it an active substance in a
pharmaceutical form?
What effects of the
medicine are you looking
for?
If you answer no to all the
questions in column C the
activity is not a clinical trial
under the scope of SI 1031.
If you answer yes to any of
the questions below go to
column D.
C.1 To discover or
verify/compare its
clinical effects?
C.2 To discover or
verify/compare its
pharmacological
effects, e.g.
pharmacodynamics?
C.3 To identify or
verify/compare its
adverse reactions?
C.4 To study or
verify/compare its
absorption, distribution,
metabolism or
excretion?
E
A NON-INTERVENTIONAL CLINICAL TRIAL?
Why are you looking
for those effects?
How are you looking for those effects?
If you answer no to all
the questions in column
D the activity is not a
clinical trial under the
scope of SI 1031.
If you answer yes to all these questions the activity is a
non-interventional trial which is outside the scope of SI
1031. If your answers in columns A,B,C & D brought
you to column E and you answer no to any of these
questions the activity is a clinical trial within the scope
of the Directive.
If you answer yes to any
of the questions below
go to column E.
D.1 To ascertain or
verify/compare the
efficacyvii of the
medicine?
D.2 To ascertain or
verify/compare the
safety of the
medicine?
E.1 Is this a study of one or more medicinal products,
which have a marketing authorisation in the UK?
E.2 Are the products prescribed in the usual manner in
accordance with the terms of that authorisation?
E.3 Does the assignment of any patient involved in the
study to a particular therapeutic strategy fall
within current practice and is not decided in
advance by a clinical trial protocolviii?
E.4 Is the decision to prescribe a particular medicinal
product clearly separated from the decision to
include the patient in the study?
E.5 Will no diagnostic or monitoring procedures be
applied to the patients included in the study, other
than those which are applied in the course of
current practice?
E.6 Will epidemiological methods be used for the
analysis of the data arising from the study?
14
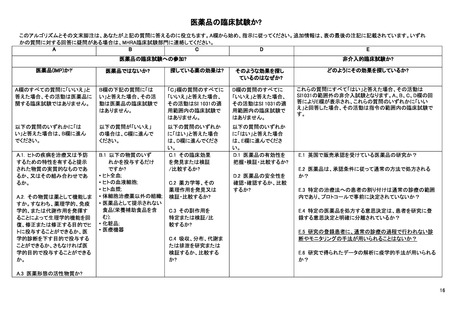
This algorithm and its endnotes will help you answer that question. Please start in column A and follow the instructions. Additional information is provided in the notes at the
end of the table. If you have doubts about the answer to any of the questions, contact the MHRA clinical trials unit.
A
B
C
D
A CLINICAL TRIAL OF A MEDICINAL PRODUCT?
Is it a medicinal product (IMP)?i
Is it not a medicinal
product?
If you answer no to all the
questions in column A, the activity
is not a clinical trial on a MP.
If you answer yes to the
question below in column
B the activity is not a
clinical trial on a MP.
If you answer yes to any of the
questions below go to column B.
If you answer no to this
question below go to
column C.
B.1 Are you only
administering any of
the following
substances?
• Human whole bloodiii;
• Human blood cells;
• Human plasma;
• Tissues except a
somatic cell therapy
medicinal productiv;
• A food product
(including dietary
supplements) not
presented as a
medicine;
• A cosmetic productvi;
• A medical device
1.1 Is it a substanceii or
combination of substances
presented as having
properties for treating or
preventing disease in human
beings?
2.2 Does the substance function
as a medicine? i.e. can it be
administered to human beings
either with a view to restoring,
correcting or modifying
physiological functions by
exerting a pharmacological,
immunological or metabolic
action or to making a medical
diagnosis or is otherwise
administered for a medicinal
purpose?
A.3 Is it an active substance in a
pharmaceutical form?
What effects of the
medicine are you looking
for?
If you answer no to all the
questions in column C the
activity is not a clinical trial
under the scope of SI 1031.
If you answer yes to any of
the questions below go to
column D.
C.1 To discover or
verify/compare its
clinical effects?
C.2 To discover or
verify/compare its
pharmacological
effects, e.g.
pharmacodynamics?
C.3 To identify or
verify/compare its
adverse reactions?
C.4 To study or
verify/compare its
absorption, distribution,
metabolism or
excretion?
E
A NON-INTERVENTIONAL CLINICAL TRIAL?
Why are you looking
for those effects?
How are you looking for those effects?
If you answer no to all
the questions in column
D the activity is not a
clinical trial under the
scope of SI 1031.
If you answer yes to all these questions the activity is a
non-interventional trial which is outside the scope of SI
1031. If your answers in columns A,B,C & D brought
you to column E and you answer no to any of these
questions the activity is a clinical trial within the scope
of the Directive.
If you answer yes to any
of the questions below
go to column E.
D.1 To ascertain or
verify/compare the
efficacyvii of the
medicine?
D.2 To ascertain or
verify/compare the
safety of the
medicine?
E.1 Is this a study of one or more medicinal products,
which have a marketing authorisation in the UK?
E.2 Are the products prescribed in the usual manner in
accordance with the terms of that authorisation?
E.3 Does the assignment of any patient involved in the
study to a particular therapeutic strategy fall
within current practice and is not decided in
advance by a clinical trial protocolviii?
E.4 Is the decision to prescribe a particular medicinal
product clearly separated from the decision to
include the patient in the study?
E.5 Will no diagnostic or monitoring procedures be
applied to the patients included in the study, other
than those which are applied in the course of
current practice?
E.6 Will epidemiological methods be used for the
analysis of the data arising from the study?
14