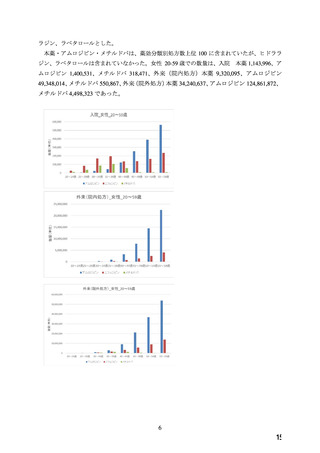
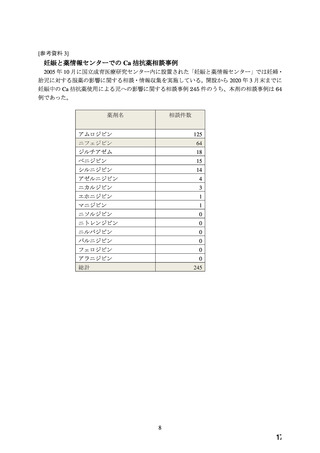
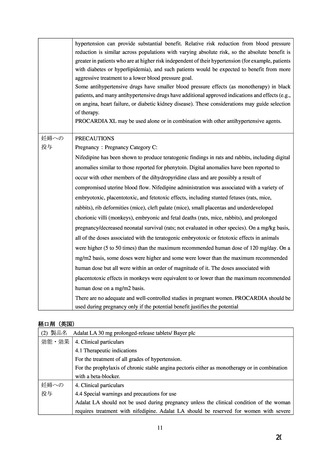
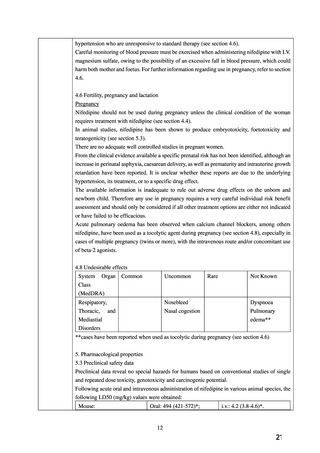
よむ、つかう、まなぶ。
資料1-3 ニフェジピン 調査結果報告書及び添付文書 (24 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29305.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第19回 11/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
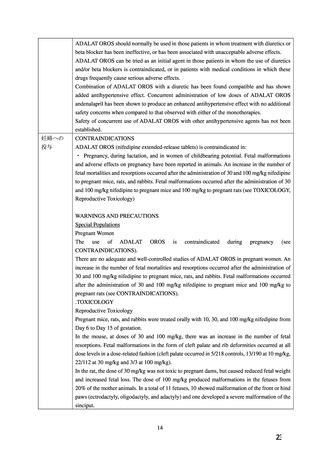
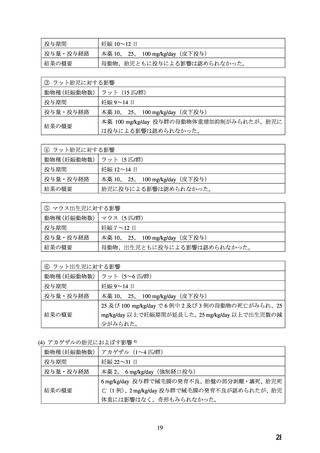
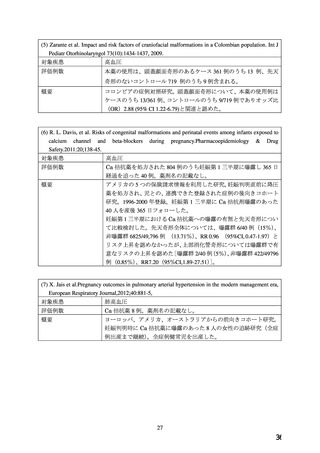
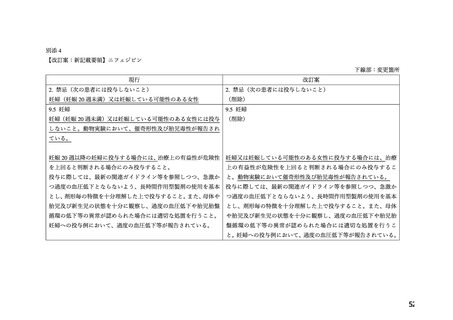
ADALAT OROS should normally be used in those patients in whom treatment with diuretics or
beta blocker has been ineffective, or has been associated with unacceptable adverse effects.
ADALAT OROS can be tried as an initial agent in those patients in whom the use of diuretics
and/or beta blockers is contraindicated, or in patients with medical conditions in which these
drugs frequently cause serious adverse effects.
Combination of ADALAT OROS with a diuretic has been found compatible and has shown
added antihypertensive effect. Concurrent administration of low doses of ADALAT OROS
andenalapril has been shown to produce an enhanced antihypertensive effect with no additional
safety concerns when compared to that observed with either of the monotherapies.
Safety of concurrent use of ADALAT OROS with other antihypertensive agents has not been
established.
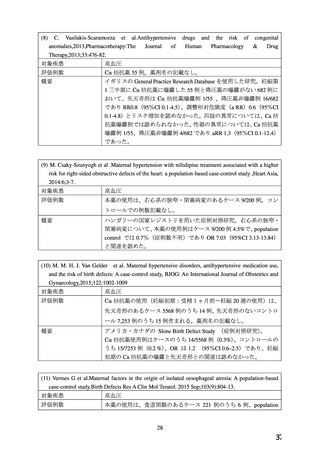
妊婦への
CONTRAINDICATIONS
投与
ADALAT OROS (nifedipine extended-release tablets) is contraindicated in:
・ Pregnancy, during lactation, and in women of childbearing potential. Fetal malformations
and adverse effects on pregnancy have been reported in animals. An increase in the number of
fetal mortalities and resorptions occurred after the administration of 30 and 100 mg/kg nifedipine
to pregnant mice, rats, and rabbits. Fetal malformations occurred after the administration of 30
and 100 mg/kg nifedipine to pregnant mice and 100 mg/kg to pregnant rats (see TOXICOLOGY,
Reproductive Toxicology)
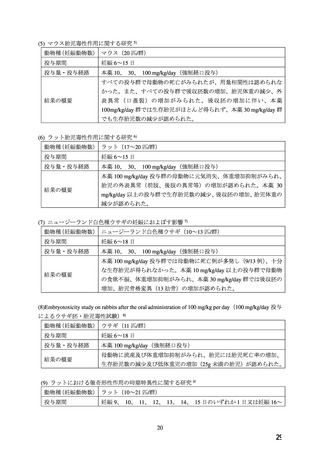
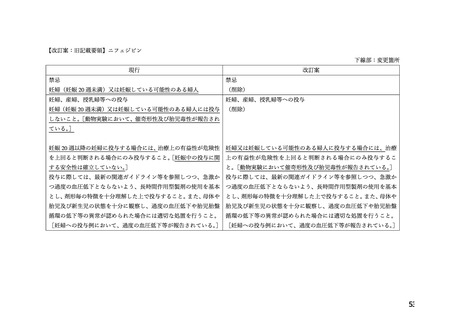
WARNINGS AND PRECAUTIONS
Special Populations
Pregnant Women
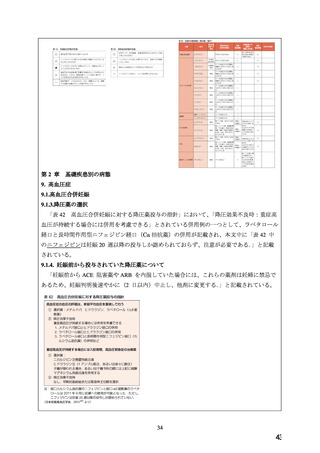
The
use
of
ADALAT
OROS
is
contraindicated
during
pregnancy
(see
CONTRAINDICATIONS).
There are no adequate and well-controlled studies of ADALAT OROS in pregnant women. An
increase in the number of fetal mortalities and resorptions occurred after the administration of
30 and 100 mg/kg nifedipine to pregnant mice, rats, and rabbits. Fetal malformations occurred
after the administration of 30 and 100 mg/kg nifedipine to pregnant mice and 100 mg/kg to
pregnant rats (see CONTRAINDICATIONS).
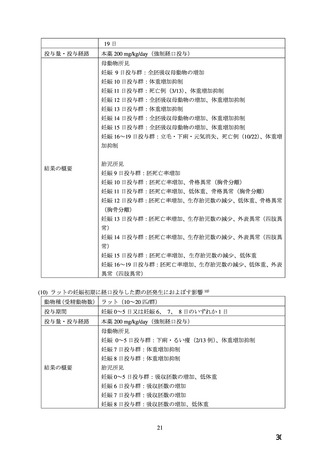
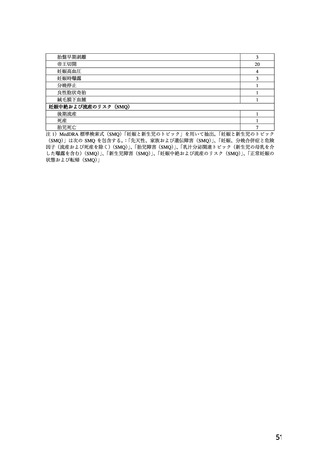
.TOXICOLOGY
Reproductive Toxicology
Pregnant mice, rats, and rabbits were treated orally with 10, 30, and 100 mg/kg nifedipine from
Day 6 to Day 15 of gestation.
In the mouse, at doses of 30 and 100 mg/kg, there was an increase in the number of fetal
resorptions. Fetal malformations in the form of cleft palate and rib deformities occurred at all
dose levels in a dose-related fashion (cleft palate occurred in 5/218 controls, 13/190 at 10 mg/kg,
22/112 at 30 mg/kg and 3/3 at 100 mg/kg).
In the rat, the dose of 30 mg/kg was not toxic to pregnant dams, but caused reduced fetal weight
and increased fetal loss. The dose of 100 mg/kg produced malformations in the fetuses from
20% of the mother animals. In a total of 11 fetuses, 10 showed malformation of the front or hind
paws (ectrodactyly, oligodactyly, and adactyly) and one developed a severe malformation of the
sinciput.
14
23
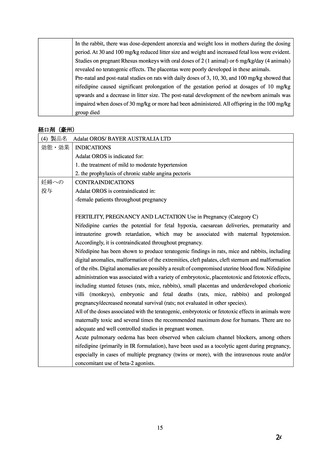
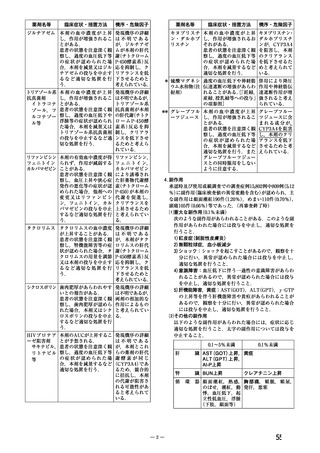
beta blocker has been ineffective, or has been associated with unacceptable adverse effects.
ADALAT OROS can be tried as an initial agent in those patients in whom the use of diuretics
and/or beta blockers is contraindicated, or in patients with medical conditions in which these
drugs frequently cause serious adverse effects.
Combination of ADALAT OROS with a diuretic has been found compatible and has shown
added antihypertensive effect. Concurrent administration of low doses of ADALAT OROS
andenalapril has been shown to produce an enhanced antihypertensive effect with no additional
safety concerns when compared to that observed with either of the monotherapies.
Safety of concurrent use of ADALAT OROS with other antihypertensive agents has not been
established.
妊婦への
CONTRAINDICATIONS
投与
ADALAT OROS (nifedipine extended-release tablets) is contraindicated in:
・ Pregnancy, during lactation, and in women of childbearing potential. Fetal malformations
and adverse effects on pregnancy have been reported in animals. An increase in the number of
fetal mortalities and resorptions occurred after the administration of 30 and 100 mg/kg nifedipine
to pregnant mice, rats, and rabbits. Fetal malformations occurred after the administration of 30
and 100 mg/kg nifedipine to pregnant mice and 100 mg/kg to pregnant rats (see TOXICOLOGY,
Reproductive Toxicology)
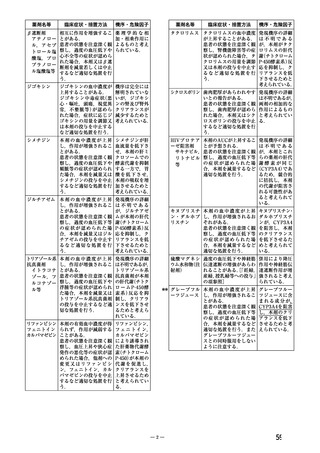
WARNINGS AND PRECAUTIONS
Special Populations
Pregnant Women
The
use
of
ADALAT
OROS
is
contraindicated
during
pregnancy
(see
CONTRAINDICATIONS).
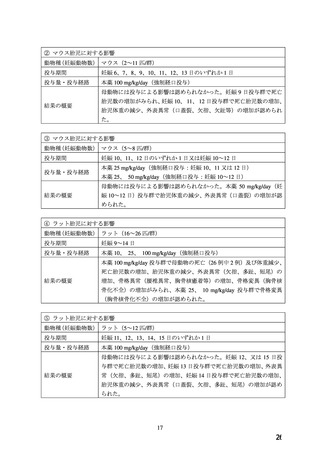
There are no adequate and well-controlled studies of ADALAT OROS in pregnant women. An
increase in the number of fetal mortalities and resorptions occurred after the administration of
30 and 100 mg/kg nifedipine to pregnant mice, rats, and rabbits. Fetal malformations occurred
after the administration of 30 and 100 mg/kg nifedipine to pregnant mice and 100 mg/kg to
pregnant rats (see CONTRAINDICATIONS).
.TOXICOLOGY
Reproductive Toxicology
Pregnant mice, rats, and rabbits were treated orally with 10, 30, and 100 mg/kg nifedipine from
Day 6 to Day 15 of gestation.
In the mouse, at doses of 30 and 100 mg/kg, there was an increase in the number of fetal
resorptions. Fetal malformations in the form of cleft palate and rib deformities occurred at all
dose levels in a dose-related fashion (cleft palate occurred in 5/218 controls, 13/190 at 10 mg/kg,
22/112 at 30 mg/kg and 3/3 at 100 mg/kg).
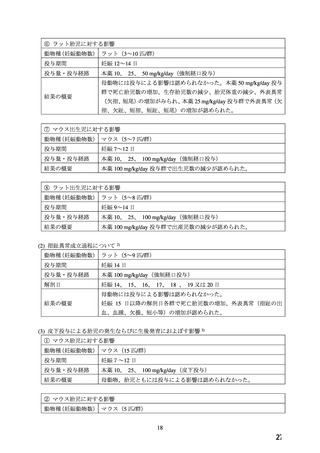
In the rat, the dose of 30 mg/kg was not toxic to pregnant dams, but caused reduced fetal weight
and increased fetal loss. The dose of 100 mg/kg produced malformations in the fetuses from
20% of the mother animals. In a total of 11 fetuses, 10 showed malformation of the front or hind
paws (ectrodactyly, oligodactyly, and adactyly) and one developed a severe malformation of the
sinciput.
14
23