よむ、つかう、まなぶ。
資料1-2 アムロジピンベシル酸塩 調査結果報告書及び添付文書 (21 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29305.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(令和4年度第19回 11/22)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
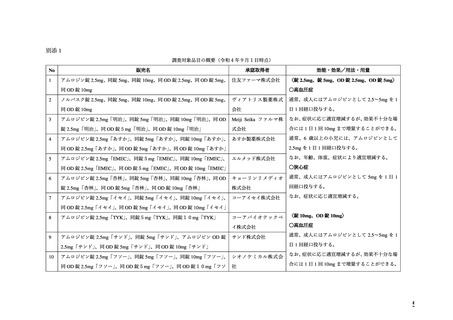
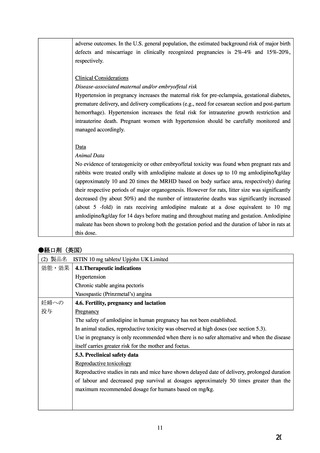
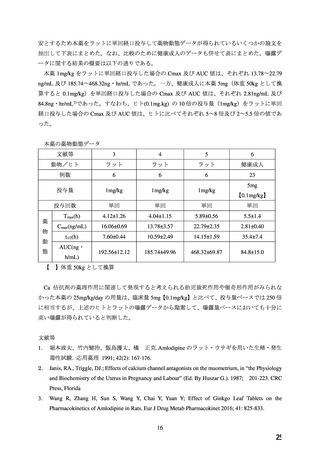
adverse outcomes. In the U.S. general population, the estimated background risk of major birth
defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%,
respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes,
premature delivery, and delivery complications (e.g., need for cesarean section and post-partum
hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and
intrauterine death. Pregnant women with hypertension should be carefully monitored and
managed accordingly.
Data
Animal Data
No evidence of teratogenicity or other embryo/fetal toxicity was found when pregnant rats and
rabbits were treated orally with amlodipine maleate at doses up to 10 mg amlodipine/kg/day
(approximately 10 and 20 times the MRHD based on body surface area, respectively) during
their respective periods of major organogenesis. However for rats, litter size was significantly
decreased (by about 50%) and the number of intrauterine deaths was significantly increased
(about 5 -fold) in rats receiving amlodipine maleate at a dose equivalent to 10 mg
amlodipine/kg/day for 14 days before mating and throughout mating and gestation. Amlodipine
maleate has been shown to prolong both the gestation period and the duration of labor in rats at
this dose.
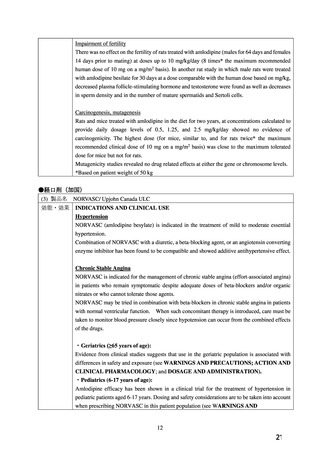
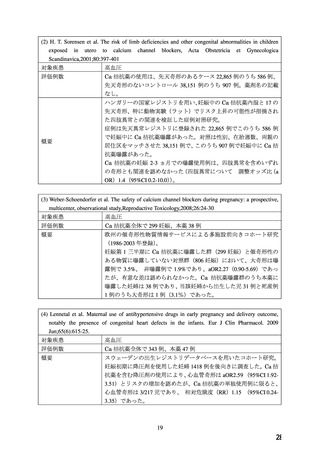
●経口剤(英国)
(2) 製品名 ISTIN 10 mg tablets/ Upjohn UK Limited
効能・効果
4.1.Therapeutic indications
Hypertension
Chronic stable angina pectoris
Vasospastic (Prinzmetal’s) angina
妊婦への
4.6. Fertility, pregnancy and lactation
投与
Pregnancy
The safety of amlodipine in human pregnancy has not been established.
In animal studies, reproductive toxicity was observed at high doses (see section 5.3).
Use in pregnancy is only recommended when there is no safer alternative and when the disease
itself carries greater risk for the mother and foetus.
5.3. Preclinical safety data
Reproductive toxicology
Reproductive studies in rats and mice have shown delayed date of delivery, prolonged duration
of labour and decreased pup survival at dosages approximately 50 times greater than the
maximum recommended dosage for humans based on mg/kg.
11
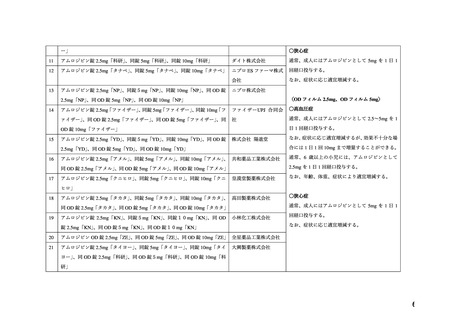
20
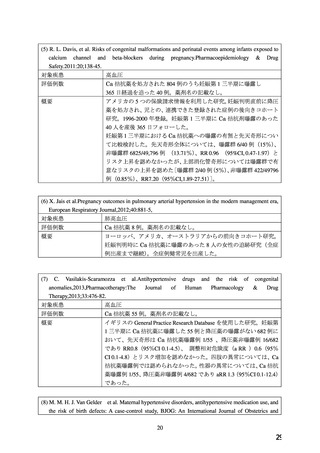
defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%,
respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes,
premature delivery, and delivery complications (e.g., need for cesarean section and post-partum
hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and
intrauterine death. Pregnant women with hypertension should be carefully monitored and
managed accordingly.
Data
Animal Data
No evidence of teratogenicity or other embryo/fetal toxicity was found when pregnant rats and
rabbits were treated orally with amlodipine maleate at doses up to 10 mg amlodipine/kg/day
(approximately 10 and 20 times the MRHD based on body surface area, respectively) during
their respective periods of major organogenesis. However for rats, litter size was significantly
decreased (by about 50%) and the number of intrauterine deaths was significantly increased
(about 5 -fold) in rats receiving amlodipine maleate at a dose equivalent to 10 mg
amlodipine/kg/day for 14 days before mating and throughout mating and gestation. Amlodipine
maleate has been shown to prolong both the gestation period and the duration of labor in rats at
this dose.
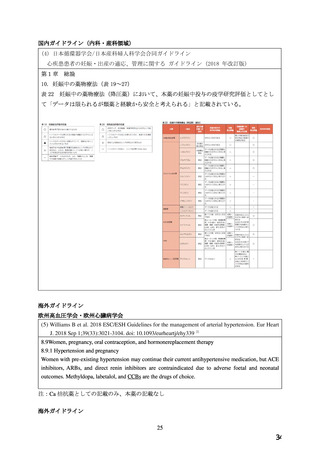
●経口剤(英国)
(2) 製品名 ISTIN 10 mg tablets/ Upjohn UK Limited
効能・効果
4.1.Therapeutic indications
Hypertension
Chronic stable angina pectoris
Vasospastic (Prinzmetal’s) angina
妊婦への
4.6. Fertility, pregnancy and lactation
投与
Pregnancy
The safety of amlodipine in human pregnancy has not been established.
In animal studies, reproductive toxicity was observed at high doses (see section 5.3).
Use in pregnancy is only recommended when there is no safer alternative and when the disease
itself carries greater risk for the mother and foetus.
5.3. Preclinical safety data
Reproductive toxicology
Reproductive studies in rats and mice have shown delayed date of delivery, prolonged duration
of labour and decreased pup survival at dosages approximately 50 times greater than the
maximum recommended dosage for humans based on mg/kg.
11
20