よむ、つかう、まなぶ。
03【資料1】新型コロナワクチンの接種について (52 ページ)
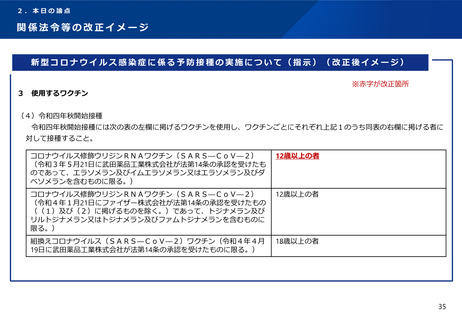
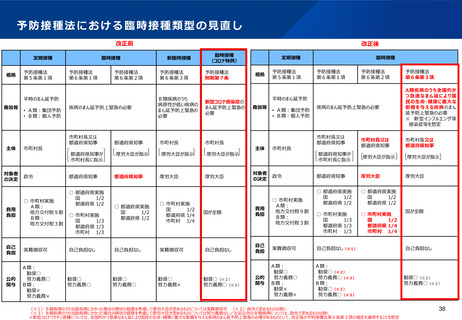
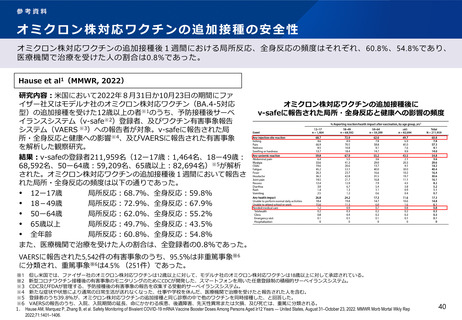
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_29727.html |
| 出典情報 | 厚生科学審議会 予防接種・ワクチン分科会(第42回 12/13)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
参考資料一覧(3/5)
諸外国における新型コロナワクチン追加接種の状況について
米国
保健福祉省 Statement by HHS Secretary Xavier Becerra on COVID-19 Vaccine Booster Doses Published Sep 24, 2021. https://www.hhs.gov/about/news/2021/09/24/statement-by-hhs-secretary-xavier-becerra-covid-19-vaccinebooster-doses.html [Accessed July 21, 2022]
CDC. COVID-19 Vaccine Booster Shots 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html [Accessed July 21, 2022].
CDC. CDC Recommends Pfizer Booster at 5 Months, Additional Primary Dose for Certain Immunocompromised Children. [online] https://www.cdc.gov/media/releases/2022/s0104-Pfizer-Booster.html. [Accessed July 21, 2022].
CDC. CDC Recommends Additional Boosters for Certain Individuals. https://www.cdc.gov/media/releases/2022/s0328-covid-19-boosters.html. [Accessed July 21, 2022].
CDC. Use of COVID-19 Vaccines in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. [Accessed July 21, 2022].
CDC. CDC Recommends Novavax’s COVID-19 Vaccine for Adults. https://www.cdc.gov/media/releases/2022/s0719-covid-novavax-vaccine.html. [Accessed 21 July 2022].
CDC. CDC Strengthens Recommendations and Expands Eligibility for COVID-19 Booster Shots. https://www.cdc.gov/media/releases/2022/s0519-covid-booster-acip.html. [Accessed 21 July 2022].
U.S. Food and Drug Administration. 2022. Coronavirus (COVID-19) Update: FDA Recommends Inclusion of Omicron BA.4/5 Component for COVID-19 Vaccine Booster Doses. [online] Available at: <https://www.fda.gov/newsevents/press-announcements/coronavirus-covid-19-update-fda-recommends-inclusion-omicron-ba45-component-covid-19-vaccine-booster> [Accessed 21 July 2022].
CDC. 2022. CDC Recommends the First Updated COVID-19 Booster. [online] Available at: <https://www.cdc.gov/media/releases/2022/s0901-covid-19-booster.html> [Accessed 13 Sep 2022].
U.S. Food and Drug Administration. 2022. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. [online] Available at: <https://www.fda.gov/newsevents/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use> [Accessed 13 Sep 2022].
CDC. 2022. CDC Expands Updated COVID-19 Vaccines to Include Children Ages 5 Through 11. [online] Available at: <https://www.cdc.gov/media/releases/2022/s1012-COVID-19-Vaccines.html> [Accessed 17 Oct 2022].
CDC. 2022. CDC Expands Updated COVID-19 Vaccines to Include Children Ages 6 Months through 5 Years. [online] Available at: <https://www.cdc.gov/media/releases/2022/s1209-covid-vaccine.html> [Accessed 12 Dec 2022].
英国
英国内閣府 COVID-19 RESPOSE: AUTUM AND WINTER PLAN Published Sep 14, 2021 https://www.gov.uk/government/publications/covid-19-response-autumn-and-winter-plan-2021/covid-19-response-autumn-and-winter-plan2021 [Accessed July 21, 2022]
Public Health England. 2021. JCVI updated advice on COVID-19 booster vaccination Sep 14, 2021 https://www.gov.uk/government/news/jcvi-issues-updated-advice-on-covid-19-booster-vaccination [Accessed July 21, 2022]
NHS. How to get a booster dose of the coronavirus (COVID-19) vaccine. https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/how-to-get-a-coronavirus-vaccine/how-to-get-a-booster-dose/ [Accessed July
21, 2022].
GOV.UK. 2022. JCVI statement on the adult COVID-19 booster vaccination programme and the Omicron variant: 7 January 2022. https://www.gov.uk/government/publications/jcvi-statement-on-the-adult-covid-19-boostervaccination-programme-and-the-omicron-variant/jcvi-statement-on-the-adult-covid-19-booster-vaccination-programme-and-the-omicron-variant-7-january-2022 [Accessed July 21, 2022].
NHS. 2022. A guide to the spring booster for those aged 75 years and older residents in care homes. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1061917/UKHSA-12308COVID-19-spring-booster-guide-for-over-75s-v2.pdf [Accessed July 21, 2022]
GOV.UK. 2022. A guide to the spring booster for those aged 75 years and older and older residents in care homes: 24 March 2022. https://www.gov.uk/government/publications/covid-19-vaccination-spring-booster-resources/aguide-to-the-spring-booster-for-those-aged-75-years-and-older-residents-in-care-homes#fn:1 [Accessed July 21, 2022].
GOV.UK. 2022. Joint Committee on Vaccination and Immunisation (JCVI) interim statement on the COVID-19 vaccination programme for autumn 2022. https://www.gov.uk/government/publications/jcvi-interim-statement-on-covid19-autumn-2022-vaccination-programme/joint-committee-on-vaccination-and-immunisation-jcvi-interim-statement-on-the-covid-19-vaccination-programme-for-autumn-2022 [Accessed Aug 4, 2022]
GOV.UK. 2022. Joint Committee on Vaccination and Immunisation (JCVI) updated statement on the COVID-19 vaccination programme for autumn 2022. https://www.gov.uk/government/publications/jcvi-updated-statement-on-thecovid-19-vaccination-programme-for-autumn-2022/joint-committee-on-vaccination-and-immunisation-jcvi-updated-statement-on-the-covid-19-vaccination-programme-for-autumn-2022 [Accessed Aug 4, 2022]
GOV.UK. 2022. JCVI publishes advice on COVID-19 vaccines for autumn booster programme. [online] Available at: <https://www.gov.uk/government/news/jcvi-publishes-advice-on-covid-19-vaccines-for-autumn-booster-programme> [Accessed Sep 1,
2022].
52
諸外国における新型コロナワクチン追加接種の状況について
米国
保健福祉省 Statement by HHS Secretary Xavier Becerra on COVID-19 Vaccine Booster Doses Published Sep 24, 2021. https://www.hhs.gov/about/news/2021/09/24/statement-by-hhs-secretary-xavier-becerra-covid-19-vaccinebooster-doses.html [Accessed July 21, 2022]
CDC. COVID-19 Vaccine Booster Shots 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html [Accessed July 21, 2022].
CDC. CDC Recommends Pfizer Booster at 5 Months, Additional Primary Dose for Certain Immunocompromised Children. [online] https://www.cdc.gov/media/releases/2022/s0104-Pfizer-Booster.html. [Accessed July 21, 2022].
CDC. CDC Recommends Additional Boosters for Certain Individuals. https://www.cdc.gov/media/releases/2022/s0328-covid-19-boosters.html. [Accessed July 21, 2022].
CDC. Use of COVID-19 Vaccines in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. [Accessed July 21, 2022].
CDC. CDC Recommends Novavax’s COVID-19 Vaccine for Adults. https://www.cdc.gov/media/releases/2022/s0719-covid-novavax-vaccine.html. [Accessed 21 July 2022].
CDC. CDC Strengthens Recommendations and Expands Eligibility for COVID-19 Booster Shots. https://www.cdc.gov/media/releases/2022/s0519-covid-booster-acip.html. [Accessed 21 July 2022].
U.S. Food and Drug Administration. 2022. Coronavirus (COVID-19) Update: FDA Recommends Inclusion of Omicron BA.4/5 Component for COVID-19 Vaccine Booster Doses. [online] Available at: <https://www.fda.gov/newsevents/press-announcements/coronavirus-covid-19-update-fda-recommends-inclusion-omicron-ba45-component-covid-19-vaccine-booster> [Accessed 21 July 2022].
CDC. 2022. CDC Recommends the First Updated COVID-19 Booster. [online] Available at: <https://www.cdc.gov/media/releases/2022/s0901-covid-19-booster.html> [Accessed 13 Sep 2022].
U.S. Food and Drug Administration. 2022. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. [online] Available at: <https://www.fda.gov/newsevents/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use> [Accessed 13 Sep 2022].
CDC. 2022. CDC Expands Updated COVID-19 Vaccines to Include Children Ages 5 Through 11. [online] Available at: <https://www.cdc.gov/media/releases/2022/s1012-COVID-19-Vaccines.html> [Accessed 17 Oct 2022].
CDC. 2022. CDC Expands Updated COVID-19 Vaccines to Include Children Ages 6 Months through 5 Years. [online] Available at: <https://www.cdc.gov/media/releases/2022/s1209-covid-vaccine.html> [Accessed 12 Dec 2022].
英国
英国内閣府 COVID-19 RESPOSE: AUTUM AND WINTER PLAN Published Sep 14, 2021 https://www.gov.uk/government/publications/covid-19-response-autumn-and-winter-plan-2021/covid-19-response-autumn-and-winter-plan2021 [Accessed July 21, 2022]
Public Health England. 2021. JCVI updated advice on COVID-19 booster vaccination Sep 14, 2021 https://www.gov.uk/government/news/jcvi-issues-updated-advice-on-covid-19-booster-vaccination [Accessed July 21, 2022]
NHS. How to get a booster dose of the coronavirus (COVID-19) vaccine. https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/how-to-get-a-coronavirus-vaccine/how-to-get-a-booster-dose/ [Accessed July
21, 2022].
GOV.UK. 2022. JCVI statement on the adult COVID-19 booster vaccination programme and the Omicron variant: 7 January 2022. https://www.gov.uk/government/publications/jcvi-statement-on-the-adult-covid-19-boostervaccination-programme-and-the-omicron-variant/jcvi-statement-on-the-adult-covid-19-booster-vaccination-programme-and-the-omicron-variant-7-january-2022 [Accessed July 21, 2022].
NHS. 2022. A guide to the spring booster for those aged 75 years and older residents in care homes. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1061917/UKHSA-12308COVID-19-spring-booster-guide-for-over-75s-v2.pdf [Accessed July 21, 2022]
GOV.UK. 2022. A guide to the spring booster for those aged 75 years and older and older residents in care homes: 24 March 2022. https://www.gov.uk/government/publications/covid-19-vaccination-spring-booster-resources/aguide-to-the-spring-booster-for-those-aged-75-years-and-older-residents-in-care-homes#fn:1 [Accessed July 21, 2022].
GOV.UK. 2022. Joint Committee on Vaccination and Immunisation (JCVI) interim statement on the COVID-19 vaccination programme for autumn 2022. https://www.gov.uk/government/publications/jcvi-interim-statement-on-covid19-autumn-2022-vaccination-programme/joint-committee-on-vaccination-and-immunisation-jcvi-interim-statement-on-the-covid-19-vaccination-programme-for-autumn-2022 [Accessed Aug 4, 2022]
GOV.UK. 2022. Joint Committee on Vaccination and Immunisation (JCVI) updated statement on the COVID-19 vaccination programme for autumn 2022. https://www.gov.uk/government/publications/jcvi-updated-statement-on-thecovid-19-vaccination-programme-for-autumn-2022/joint-committee-on-vaccination-and-immunisation-jcvi-updated-statement-on-the-covid-19-vaccination-programme-for-autumn-2022 [Accessed Aug 4, 2022]
GOV.UK. 2022. JCVI publishes advice on COVID-19 vaccines for autumn booster programme. [online] Available at: <https://www.gov.uk/government/news/jcvi-publishes-advice-on-covid-19-vaccines-for-autumn-booster-programme> [Accessed Sep 1,
2022].
52