よむ、つかう、まなぶ。
参考資料13 Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting Presentation - FDA Review of Effectiveness and Safety of Novavax COVID-19 Vaccine in Adults > 18 Years of Age - Emergency Use Authorization Request(Vaccines and Related Biological Products Advisory Committee June 7, 2022 Meeting FDA提出資料) (39 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000208910_00043.html |
| 出典情報 | 第80回厚生科学審議会予防接種・ワクチン分科会副反応検討部会、令和4年度第5回薬事・食品衛生審議会薬事分科会医薬品等安全対策部会安全対策調査会(合同開催)(6/10)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
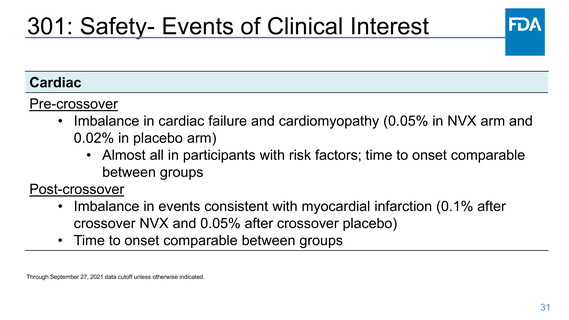
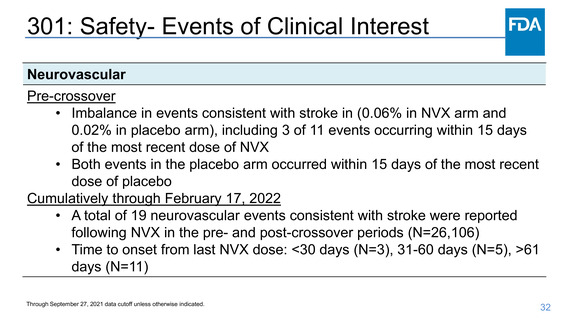
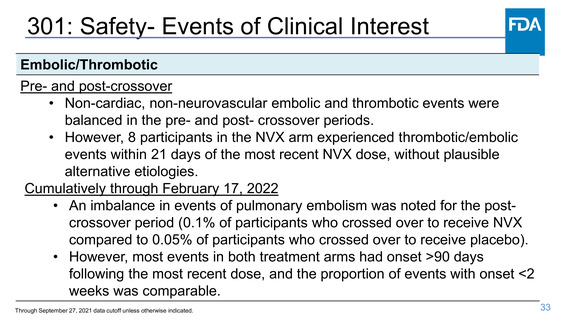
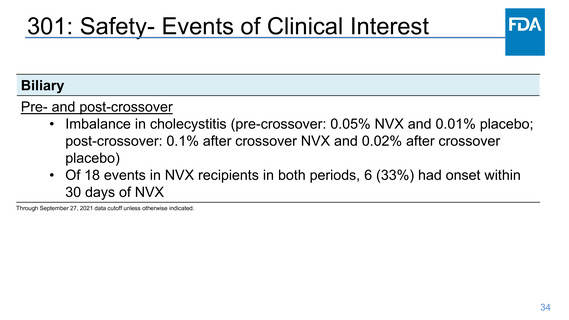
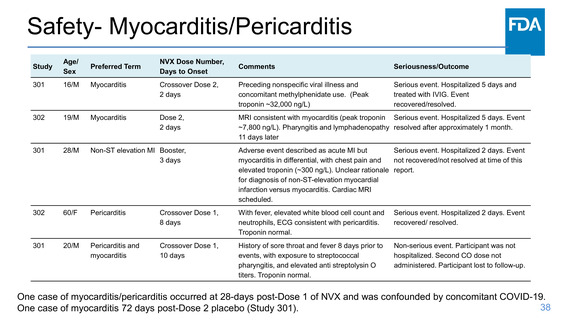
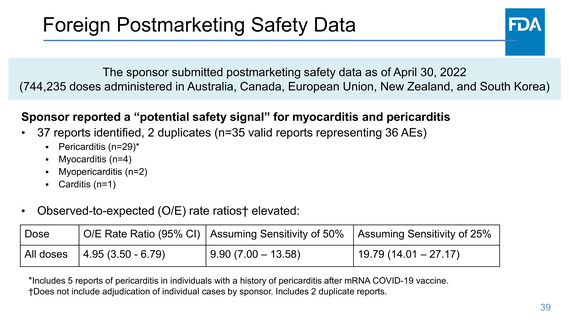
Safety- Myocarditis/Pericarditis
Study
Age/
Sex
Preferred Term
301
16/M
Myocarditis
302
19/M
Myocarditis
301
NVX Dose Number,
Days to Onset
Comments
Seriousness/Outcome
Crossover Dose 2,
2 days
Preceding nonspecific viral illness and
concomitant methylphenidate use. (Peak
troponin ~32,000 ng/L)
Serious event. Hospitalized 5 days and
treated with IVIG. Event
recovered/resolved.
Dose 2,
2 days
MRI consistent with myocarditis (peak troponin Serious event. Hospitalized 5 days. Event
~7,800 ng/L). Pharyngitis and lymphadenopathy resolved after approximately 1 month.
11 days later
28/M
Non-ST elevation MI Booster,
3 days
Adverse event described as acute MI but
Serious event. Hospitalized 2 days. Event
myocarditis in differential, with chest pain and
not recovered/not resolved at time of this
elevated troponin (~300 ng/L). Unclear rationale report.
for diagnosis of non-ST-elevation myocardial
infarction versus myocarditis. Cardiac MRI
scheduled.
302
60/F
Pericarditis
Crossover Dose 1,
8 days
With fever, elevated white blood cell count and
neutrophils, ECG consistent with pericarditis.
Troponin normal.
Serious event. Hospitalized 2 days. Event
recovered/ resolved.
301
20/M
Pericarditis and
myocarditis
Crossover Dose 1,
10 days
History of sore throat and fever 8 days prior to
events, with exposure to streptococcal
pharyngitis, and elevated anti streptolysin O
titers. Troponin normal.
Non-serious event. Participant was not
hospitalized. Second CO dose not
administered. Participant lost to follow-up.
One case of myocarditis/pericarditis occurred at 28-days post-Dose 1 of NVX and was confounded by concomitant COVID-19.
38
One case of myocarditis 72 days post-Dose 2 placebo (Study 301).
Study
Age/
Sex
Preferred Term
301
16/M
Myocarditis
302
19/M
Myocarditis
301
NVX Dose Number,
Days to Onset
Comments
Seriousness/Outcome
Crossover Dose 2,
2 days
Preceding nonspecific viral illness and
concomitant methylphenidate use. (Peak
troponin ~32,000 ng/L)
Serious event. Hospitalized 5 days and
treated with IVIG. Event
recovered/resolved.
Dose 2,
2 days
MRI consistent with myocarditis (peak troponin Serious event. Hospitalized 5 days. Event
~7,800 ng/L). Pharyngitis and lymphadenopathy resolved after approximately 1 month.
11 days later
28/M
Non-ST elevation MI Booster,
3 days
Adverse event described as acute MI but
Serious event. Hospitalized 2 days. Event
myocarditis in differential, with chest pain and
not recovered/not resolved at time of this
elevated troponin (~300 ng/L). Unclear rationale report.
for diagnosis of non-ST-elevation myocardial
infarction versus myocarditis. Cardiac MRI
scheduled.
302
60/F
Pericarditis
Crossover Dose 1,
8 days
With fever, elevated white blood cell count and
neutrophils, ECG consistent with pericarditis.
Troponin normal.
Serious event. Hospitalized 2 days. Event
recovered/ resolved.
301
20/M
Pericarditis and
myocarditis
Crossover Dose 1,
10 days
History of sore throat and fever 8 days prior to
events, with exposure to streptococcal
pharyngitis, and elevated anti streptolysin O
titers. Troponin normal.
Non-serious event. Participant was not
hospitalized. Second CO dose not
administered. Participant lost to follow-up.
One case of myocarditis/pericarditis occurred at 28-days post-Dose 1 of NVX and was confounded by concomitant COVID-19.
38
One case of myocarditis 72 days post-Dose 2 placebo (Study 301).