よむ、つかう、まなぶ。
資料4-1 オキサリプラチン (21 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/shingi2/0000198856_00024.html |
| 出典情報 | 医療上の必要性の高い未承認薬・適応外薬検討会議(第52回 8/31)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
7) Enzinger PC, et al. CALGB 80403 (Alliance)/E1206: A Randomized Phase II Study of Three
Chemotherapy Regimens Plus Cetuximab in Metastatic Esophageal and Gastroesophageal
Junction Cancers. J Clin Oncol 2016; 34: 2736-42.
8) Blum Murphy MA, et al. A phase I/II study of docetaxel, oxaliplatin, and fluorouracil (D-FOX)
chemotherapy in patients with untreated locally unresectable or metastatic adenocarcinoma of
the stomach and gastroesophageal Junction. Am J Clin Oncol 2018; 41: 321-5.
9) Smyth EC, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol 2016; 27: v38-49.
10) Catalano V, et al. A phase II study of modified FOLFOX as first-line chemotherapy
for metastatic gastric cancer in elderly patients with associated diseases. Gastric Cancer 2013;
16: 411-9.
11) Alberta Health services clinical practice guidelineGI-008 Version 6
12) Iqbal S, et al. Randomized, phase II study prospectively evaluating treatment of metastatic
esophageal, gastric, or gastroesophageal cancer by gene expression of ERCC1: SWOG S1201.
J Clin Oncol 2020; 38: 472-9.
13) Shah MA, et al.Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without
Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The
METGastric Randomized Clinical Trial. JAMA Oncol 2017; 3: 620-7.
14) Yoon HH, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced
esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, doubleblind, multicenter Phase II trial. Ann Oncol 2016; 27: 2196-203.
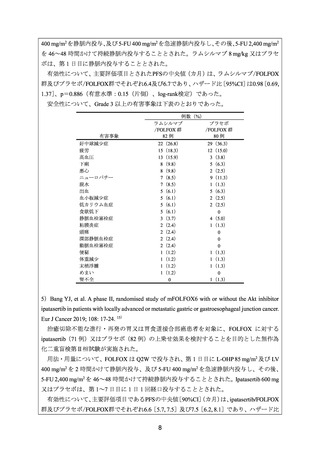
15) Bang YJ, et al. A phase II, randomised study of mFOLFOX6 with or without the Akt inhibitor
ipatasertib in patients with locally advanced or metastatic gastric or gastroesophageal junction
cancer. Eur J Cancer 2019; 108: 17-24.
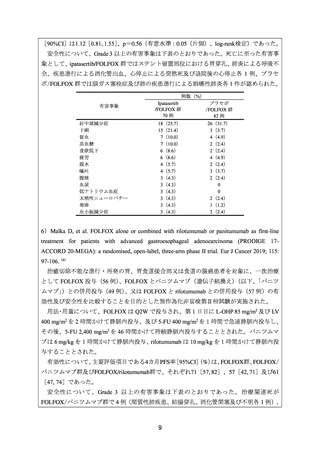
16) Malka D, et al. FOLFOX alone or combined with rilotumumab or panitumumab as first-line
treatment for patients with advanced gastroesophageal adenocarcinoma (PRODIGE 17ACCORD 20-MEGA): a randomised, open-label, three-arm phase II trial. Eur J Cancer 2019;
115: 97-106.
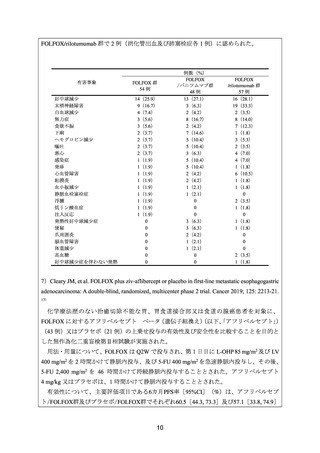
17) Cleary JM, et al. FOLFOX plus ziv-aflibercept or placebo in first-line metastatic esophagogastric
adenocarcinoma: A double-blind, randomized, multicenter phase 2 trial. Cancer 2019;
125: 2213-21.
18) Mitani S, et al. A Phase II Study of Modified FOLFOX6 for Advanced Gastric Cancer Refractory
to Standard Therapies. Adv Ther 2020; 37: 2853-64.
19) 船坂知華子, 他 進行再発胃癌に対する Modified FOLFOX6 療法の有効性に関する検討.
癌と化学療法 2020; 47: 49-53.
20) Kondoh C, et al. Salvage chemotherapy with the combination of oxaliplatin, leucovorin, and 5fluorouracil in advanced gastric cancer refractory or intolerant to fluoropyrimidines, platinum,
21
Chemotherapy Regimens Plus Cetuximab in Metastatic Esophageal and Gastroesophageal
Junction Cancers. J Clin Oncol 2016; 34: 2736-42.
8) Blum Murphy MA, et al. A phase I/II study of docetaxel, oxaliplatin, and fluorouracil (D-FOX)
chemotherapy in patients with untreated locally unresectable or metastatic adenocarcinoma of
the stomach and gastroesophageal Junction. Am J Clin Oncol 2018; 41: 321-5.
9) Smyth EC, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol 2016; 27: v38-49.
10) Catalano V, et al. A phase II study of modified FOLFOX as first-line chemotherapy
for metastatic gastric cancer in elderly patients with associated diseases. Gastric Cancer 2013;
16: 411-9.
11) Alberta Health services clinical practice guidelineGI-008 Version 6
12) Iqbal S, et al. Randomized, phase II study prospectively evaluating treatment of metastatic
esophageal, gastric, or gastroesophageal cancer by gene expression of ERCC1: SWOG S1201.
J Clin Oncol 2020; 38: 472-9.
13) Shah MA, et al.Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without
Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The
METGastric Randomized Clinical Trial. JAMA Oncol 2017; 3: 620-7.
14) Yoon HH, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced
esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, doubleblind, multicenter Phase II trial. Ann Oncol 2016; 27: 2196-203.
15) Bang YJ, et al. A phase II, randomised study of mFOLFOX6 with or without the Akt inhibitor
ipatasertib in patients with locally advanced or metastatic gastric or gastroesophageal junction
cancer. Eur J Cancer 2019; 108: 17-24.
16) Malka D, et al. FOLFOX alone or combined with rilotumumab or panitumumab as first-line
treatment for patients with advanced gastroesophageal adenocarcinoma (PRODIGE 17ACCORD 20-MEGA): a randomised, open-label, three-arm phase II trial. Eur J Cancer 2019;
115: 97-106.
17) Cleary JM, et al. FOLFOX plus ziv-aflibercept or placebo in first-line metastatic esophagogastric
adenocarcinoma: A double-blind, randomized, multicenter phase 2 trial. Cancer 2019;
125: 2213-21.
18) Mitani S, et al. A Phase II Study of Modified FOLFOX6 for Advanced Gastric Cancer Refractory
to Standard Therapies. Adv Ther 2020; 37: 2853-64.
19) 船坂知華子, 他 進行再発胃癌に対する Modified FOLFOX6 療法の有効性に関する検討.
癌と化学療法 2020; 47: 49-53.
20) Kondoh C, et al. Salvage chemotherapy with the combination of oxaliplatin, leucovorin, and 5fluorouracil in advanced gastric cancer refractory or intolerant to fluoropyrimidines, platinum,
21