よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (46 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_38751.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第20回 3/18)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
When therapeutic interventions are actually carried out on patients,
confirmation tests will be carried out using other analysis methods with
guaranteed accuracy (different types of genetic tests, companion
diagnostics, cancer gene panel tests, etc.).
o Evaluation of the results of whole genome analysis
As a technical issue, the analytical validity of analysis results obtained
by whole genome analysis is unknown at this stage. The analytical
validity and clinical usefulness of the results will therefore be evaluated
by comparing them with already confirmed cancer gene panel tests, etc.
to consider how the confirmation tests and whole genome analysis
should be conducted to better benefit patients.
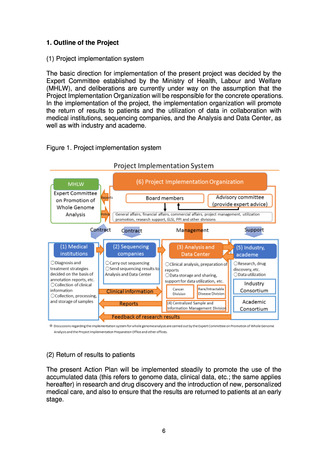
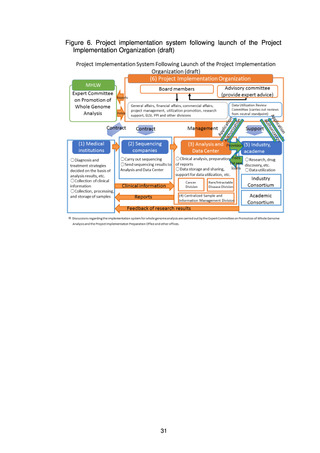
(2) Utilization
It would be desirable to promptly put in place a system allowing fair, safe utilization
of data and samples collected through the present project to promote research and
development for drug discovery and diagnostic technologies.
The Analysis and Data Center will share data with the Industry Consortium and
the Academic Consortium on the basis of the data utilization policy and the rules on
data sharing and will also promote utilization of the data using the data sharing
system (research support system). In addition, a Data Utilization Review Committee
established within the Project Implementation Organization will review applications
from users for uses that require an application to be made, such as detailed analysis,
and will decide whether to grant a license for use.
The Project Implementation Preparation Office will formulate the data utilization
policy and the rules for data sharing, hold discussions on establishment of the Data
Utilization Review Committee within the Project Implementation Organization, and
conduct pilot operations during FY2022, with the aim of commencing full-scale data
sharing during FY2023.
(a) Data utilization policy
The data utilization policy stipulates the following for fair, seamless utilization of
data.
o Basic concepts for data utilization
• Utilization of the data shall be limited to academic research, development
of pharmaceutical products, etc., and utilization for preventive purposes
based on scientific evidence.
• The users shall be domestic and foreign companies and academic research
institutions belonging to the Industry Consortium or Academic Consortium.
However, for use outside Japan, users must be from countries or regions
with systems to protect personal information that are recognized as being
of a similar level to those in Japan.
• In the event that a user violates the data utilization policy, the Project
Implementation Organization may take measures such as publicizing the
user’s name, suspending their license to use data, refusing new
applications to use data, seeking an injunction, or claiming compensation
for damages.
45
confirmation tests will be carried out using other analysis methods with
guaranteed accuracy (different types of genetic tests, companion
diagnostics, cancer gene panel tests, etc.).
o Evaluation of the results of whole genome analysis
As a technical issue, the analytical validity of analysis results obtained
by whole genome analysis is unknown at this stage. The analytical
validity and clinical usefulness of the results will therefore be evaluated
by comparing them with already confirmed cancer gene panel tests, etc.
to consider how the confirmation tests and whole genome analysis
should be conducted to better benefit patients.
(2) Utilization
It would be desirable to promptly put in place a system allowing fair, safe utilization
of data and samples collected through the present project to promote research and
development for drug discovery and diagnostic technologies.
The Analysis and Data Center will share data with the Industry Consortium and
the Academic Consortium on the basis of the data utilization policy and the rules on
data sharing and will also promote utilization of the data using the data sharing
system (research support system). In addition, a Data Utilization Review Committee
established within the Project Implementation Organization will review applications
from users for uses that require an application to be made, such as detailed analysis,
and will decide whether to grant a license for use.
The Project Implementation Preparation Office will formulate the data utilization
policy and the rules for data sharing, hold discussions on establishment of the Data
Utilization Review Committee within the Project Implementation Organization, and
conduct pilot operations during FY2022, with the aim of commencing full-scale data
sharing during FY2023.
(a) Data utilization policy
The data utilization policy stipulates the following for fair, seamless utilization of
data.
o Basic concepts for data utilization
• Utilization of the data shall be limited to academic research, development
of pharmaceutical products, etc., and utilization for preventive purposes
based on scientific evidence.
• The users shall be domestic and foreign companies and academic research
institutions belonging to the Industry Consortium or Academic Consortium.
However, for use outside Japan, users must be from countries or regions
with systems to protect personal information that are recognized as being
of a similar level to those in Japan.
• In the event that a user violates the data utilization policy, the Project
Implementation Organization may take measures such as publicizing the
user’s name, suspending their license to use data, refusing new
applications to use data, seeking an injunction, or claiming compensation
for damages.
45