よむ、つかう、まなぶ。
参考資料4_Action plan for whole genome analysis 2022 (28 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_38751.html |
| 出典情報 | 厚生科学審議会 科学技術部会全ゲノム解析等の推進に関する専門委員会(第20回 3/18)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
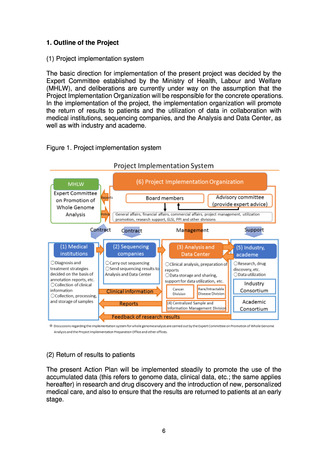
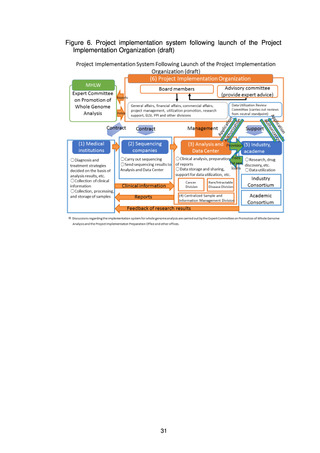
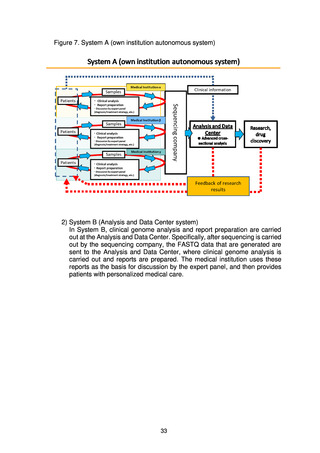
4) Development of human resources
Operating, maintaining, and improving the Analysis and Data Center will
require the involvement of personnel with a wide diversity of specialized skills,
and systems will therefore be examined to develop and secure human
resources for the Analysis and Data Center through collaboration with
graduate schools and personnel exchanges with academe and industry.
In particular, for the development of experts in information analysis and
artificial intelligence for genome analysis, educational seminars, etc. will be
held to widely disseminate knowledge related to genome analysis in
collaboration with the Project for Development of Human Resources Relating
to Whole Genome Analysis of Cancer (tentative name) being implemented by
the MHLW, and will also develop human resources through on-the-job training
(OJT) at divisions that conduct genome analysis.
(d) Centralized Sample and Information Management Division
A Centralized Sample and Information Management Division will be set up
within the Analysis and Data Center for centralized management and
utilization of whole genome data, clinical information, samples, and
information on samples. A prototype system for the development of the
division’s centralized management system will be completed in FY2022.
In addition, there is a need to establish a sample transfer system that
allows third parties not only to use genome data, but also, when necessary, to
carry out omics analyses that combine samples of tissue, etc. (surplus
samples and residual samples) with genome information and clinical
information. The sample management system for this will be created and
operated as follows.
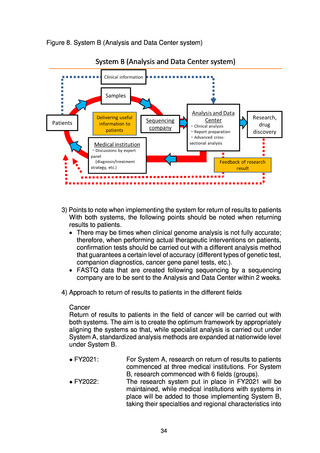
o Sample management system (Centralized Sample Management Center)
and rules for storage and management
For samples from new patients, a system will be established to enable
collective management using existing facilities. At the same time, samples
can be stored at individual medical institutions as long as the quality of the
storage and management is the same as that of the collective management,
and if necessary, a system is in place allowing samples to be transferred
through the same procedures.
In addition, a system will be established to enable the Centralized
Sample Management Center to grasp the type of samples, the amount
remaining, and the contents of consent (if sample transfer only to industry
is possible, etc.), which will also include samples stored at individual
medical institutions.
These systems will be established on a trial basis during FY2022, with
the aim of full-scale operation from FY2023 onward.
In addition, standard operating procedures for sample storage and
management rules (detailed SOPs for each organ) will be developed during
FY2022 with the cooperation of experts from the Japan Registered Clinical
Laboratories Association, to comply with international standards.
(e) Industry Consortium, Academic Consortium
Consortiums in which industry and academe can participate will be formed
and the industry-academic collaborative utilization of data will be promoted to
27
Operating, maintaining, and improving the Analysis and Data Center will
require the involvement of personnel with a wide diversity of specialized skills,
and systems will therefore be examined to develop and secure human
resources for the Analysis and Data Center through collaboration with
graduate schools and personnel exchanges with academe and industry.
In particular, for the development of experts in information analysis and
artificial intelligence for genome analysis, educational seminars, etc. will be
held to widely disseminate knowledge related to genome analysis in
collaboration with the Project for Development of Human Resources Relating
to Whole Genome Analysis of Cancer (tentative name) being implemented by
the MHLW, and will also develop human resources through on-the-job training
(OJT) at divisions that conduct genome analysis.
(d) Centralized Sample and Information Management Division
A Centralized Sample and Information Management Division will be set up
within the Analysis and Data Center for centralized management and
utilization of whole genome data, clinical information, samples, and
information on samples. A prototype system for the development of the
division’s centralized management system will be completed in FY2022.
In addition, there is a need to establish a sample transfer system that
allows third parties not only to use genome data, but also, when necessary, to
carry out omics analyses that combine samples of tissue, etc. (surplus
samples and residual samples) with genome information and clinical
information. The sample management system for this will be created and
operated as follows.
o Sample management system (Centralized Sample Management Center)
and rules for storage and management
For samples from new patients, a system will be established to enable
collective management using existing facilities. At the same time, samples
can be stored at individual medical institutions as long as the quality of the
storage and management is the same as that of the collective management,
and if necessary, a system is in place allowing samples to be transferred
through the same procedures.
In addition, a system will be established to enable the Centralized
Sample Management Center to grasp the type of samples, the amount
remaining, and the contents of consent (if sample transfer only to industry
is possible, etc.), which will also include samples stored at individual
medical institutions.
These systems will be established on a trial basis during FY2022, with
the aim of full-scale operation from FY2023 onward.
In addition, standard operating procedures for sample storage and
management rules (detailed SOPs for each organ) will be developed during
FY2022 with the cooperation of experts from the Japan Registered Clinical
Laboratories Association, to comply with international standards.
(e) Industry Consortium, Academic Consortium
Consortiums in which industry and academe can participate will be formed
and the industry-academic collaborative utilization of data will be promoted to
27