よむ、つかう、まなぶ。
資料1-3 海外添付文書の記載状況 (4 ページ)
出典
| 公開元URL | https://www.mhlw.go.jp/stf/newpage_23462.html |
| 出典情報 | 薬事・食品衛生審議会 薬事分科会医薬品等安全対策部会安全対策調査会(第27回 1/24)《厚生労働省》 |
ページ画像
ダウンロードした画像を利用する際は「出典情報」を明記してください。
低解像度画像をダウンロード
プレーンテキスト
資料テキストはコンピュータによる自動処理で生成されており、完全に資料と一致しない場合があります。
テキストをコピーしてご利用いただく際は資料と付け合わせてご確認ください。
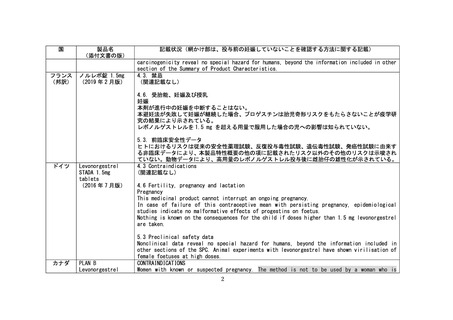
国
製品名
(添付文書の版)
記載状況(網かけ部は、投与前の妊娠していないことを確認する方法に関する記載)
Segment II studies were given by repeat dose during organogenesis. At the levels required to
maintain pregnancy, virilizing effects were noted and were considerably greater than those
of progesterone. Two of 439 fetuses from dams treated at these levels of norgestrel were
deformed;
one fetus from a dam treated with norgestrel 3 mg subcutaneously had incomplete spinal closure
and one fetus from a dam treated with norgestrel 3 mg orally had a poorly developed cranium.
The 88 control fetuses were normal. Occasional deformities appeared in the other progestogen
groups, and were more frequent from spayed mothers. In a study where norgestrel was given
subcutaneously from Days 16 to 19 of gestation, potency in producing virilization in female
fetuses was found to be nearly equal to testosterone propionate and three times greater than
norethindrone acetate. Histological examination showed that norgestrel 0.1 mg/day
subcutaneously was effective, while 10 mg/day orally was ineffective. For a macroscopically
detectable increase in ano-genital distance, 3 mg/day subcutaneously was required.
オースト
ラリア
NorLevo-1
levonorgestrel 1.5
mg tablet
(2020 年 1 月版)
4.3 CONTRAINDICATIONS
NorLevo-1 should not be given to pregnant women. If menstrual bleeding is overdue, if the
last menstrual period was abnormal in timing or character or if pregnancy is suspected for
any other reason, pregnancy should be excluded (by pregnancy testing or pelvic examination)
before treatment is given.
If a woman has had unprotected intercourse more than 72 hours earlier in the same menstrual
cycle conception may have already occurred. Treatment with NorLevo-1 following the second act
of intercourse may therefore be ineffective in preventing pregnancy. While the consensus is
that levonorgestrel is not teratogenic, no guarantee can be given that pregnancy will result
in a normal baby.
4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE
Precautions before use
Exclude pregnancy if suspected clinically.
Breast or pelvic examinations are not routinely necessary. Perform such examinations only if
indicated by the patient's history.
Blood pressure may be measured before recommending NorLevo-1. An elevated blood pressure is
not a contraindication to treatment but indicates the need for further investigation.
4
製品名
(添付文書の版)
記載状況(網かけ部は、投与前の妊娠していないことを確認する方法に関する記載)
Segment II studies were given by repeat dose during organogenesis. At the levels required to
maintain pregnancy, virilizing effects were noted and were considerably greater than those
of progesterone. Two of 439 fetuses from dams treated at these levels of norgestrel were
deformed;
one fetus from a dam treated with norgestrel 3 mg subcutaneously had incomplete spinal closure
and one fetus from a dam treated with norgestrel 3 mg orally had a poorly developed cranium.
The 88 control fetuses were normal. Occasional deformities appeared in the other progestogen
groups, and were more frequent from spayed mothers. In a study where norgestrel was given
subcutaneously from Days 16 to 19 of gestation, potency in producing virilization in female
fetuses was found to be nearly equal to testosterone propionate and three times greater than
norethindrone acetate. Histological examination showed that norgestrel 0.1 mg/day
subcutaneously was effective, while 10 mg/day orally was ineffective. For a macroscopically
detectable increase in ano-genital distance, 3 mg/day subcutaneously was required.
オースト
ラリア
NorLevo-1
levonorgestrel 1.5
mg tablet
(2020 年 1 月版)
4.3 CONTRAINDICATIONS
NorLevo-1 should not be given to pregnant women. If menstrual bleeding is overdue, if the
last menstrual period was abnormal in timing or character or if pregnancy is suspected for
any other reason, pregnancy should be excluded (by pregnancy testing or pelvic examination)
before treatment is given.
If a woman has had unprotected intercourse more than 72 hours earlier in the same menstrual
cycle conception may have already occurred. Treatment with NorLevo-1 following the second act
of intercourse may therefore be ineffective in preventing pregnancy. While the consensus is
that levonorgestrel is not teratogenic, no guarantee can be given that pregnancy will result
in a normal baby.
4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE
Precautions before use
Exclude pregnancy if suspected clinically.
Breast or pelvic examinations are not routinely necessary. Perform such examinations only if
indicated by the patient's history.
Blood pressure may be measured before recommending NorLevo-1. An elevated blood pressure is
not a contraindication to treatment but indicates the need for further investigation.
4